Oxidation-Reduction Reactions
Science
half-reaction
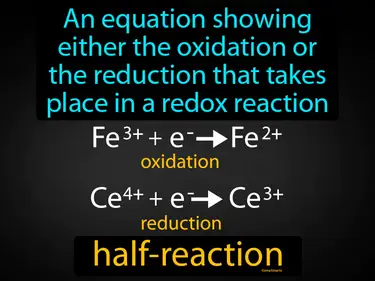
An equation showing either the oxidation or the reduction that takes place in a redox reaction. Half-reaction. A half-reaction is a part of a redox reaction that shows either the loss or gain of electrons.
oxidation number
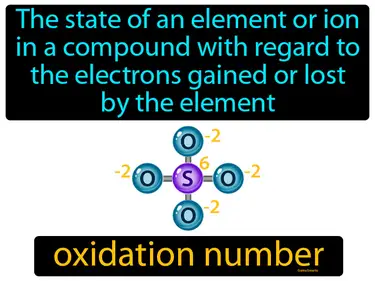
The state of an element or ion in a compound with regard to the electrons gained or lost by the element. Oxidation number. It is a number assigned to an element in a chemical compound that represents the number of electrons lost or gained by an atom of that element.
oxidation-reduction reaction
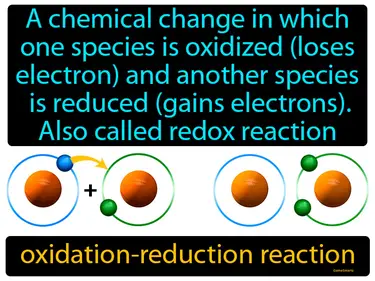
A chemical change in which one species is oxidized loses electrons and another species is reduced gains electrons, also called redox reaction. Oxidation-reduction reaction. In simple terms, a redox reaction is when one substance donates electrons to another, changing their electron counts.
oxidizing agent
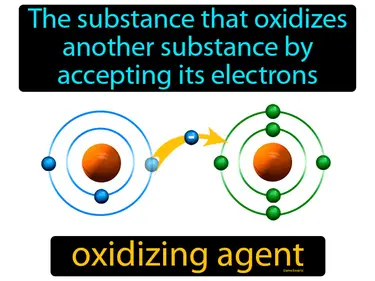
The substance that oxidizes another substance by accepting its electrons. Oxidizing agent. An oxidizing agent gains electrons and causes another substance to lose them.
reducing agent
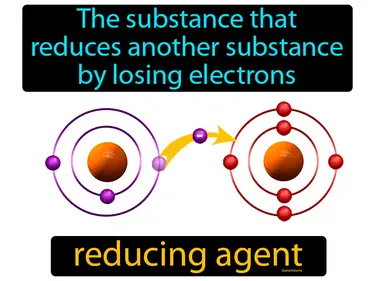
The substance that reduces another substance by losing electrons reducing agent. A reducing agent is a chemical that donates electrons to another substance in a reaction.
reduction
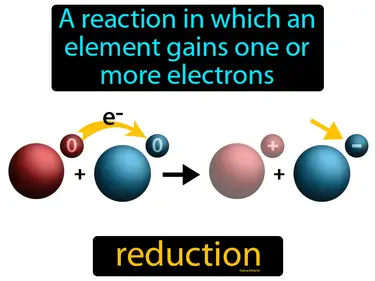
A reaction in which an element gains one or more electrons. Reduction. In simple terms, reduction is the process where a substance gains electrons during a chemical reaction.

