Oxidation Number
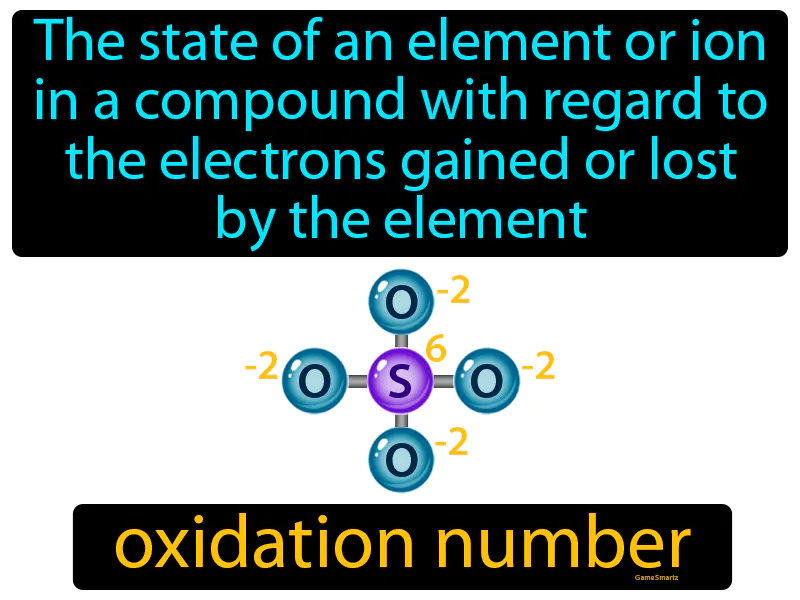
Imagine you're trying to balance your monthly budget, deciding how much money you need to allocate for necessities, savings, and leisure. This scenario is similar to an element or ion in a compound determining its oxidation number because it's about how much "currency" (electrons or dollars) is gained or lost. Just as balancing your budget ensures you allocate the right amount of money to different categories, determining the oxidation number involves accounting for the electrons gained or lost to maintain balance within a compound.
Practice Version
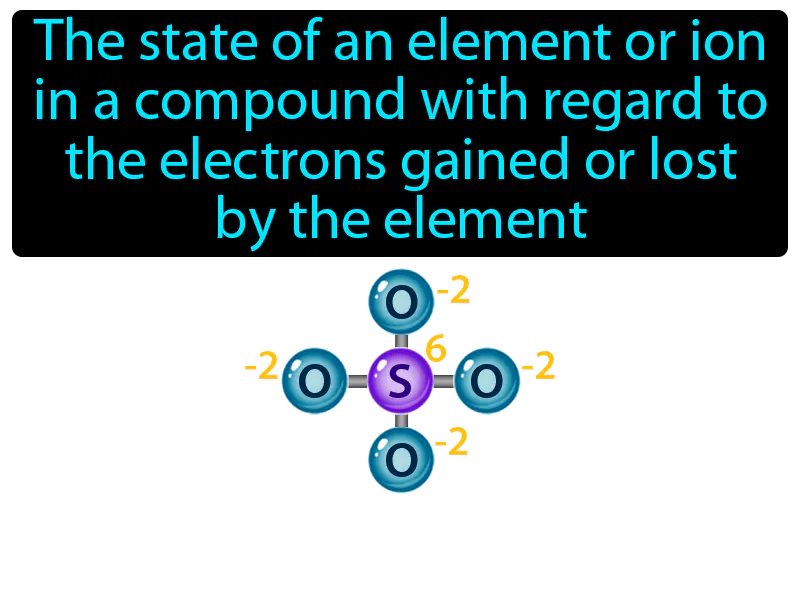
Oxidation Number: The state of an element or ion in a compound with regard to the electrons gained or lost by the element. Oxidation number. It is a number assigned to an element in a chemical compound that represents the number of electrons lost or gained by an atom of that element.