Ionic and Metallic Bonding
Science
alloy
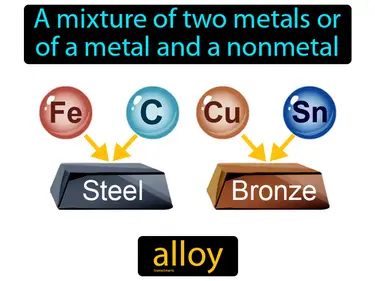
A mixture of two metals or of a metal and a nonmetal. Alloy. An alloy is a blend of different metals designed to enhance strength or other properties.
anhydrous

A substance from which all water has been removed. Anhydrous. In simple terms, "anhydrous" means a material that does not contain any water.
anion
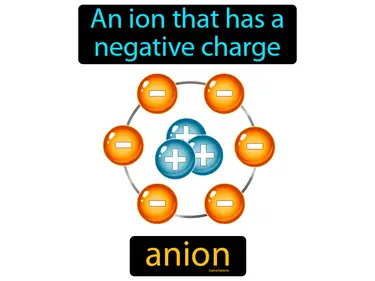
An ion that has a negative charge. Anion. An anion is an atom or molecule that has gained one or more extra electrons, giving it a negative charge.
binary compound
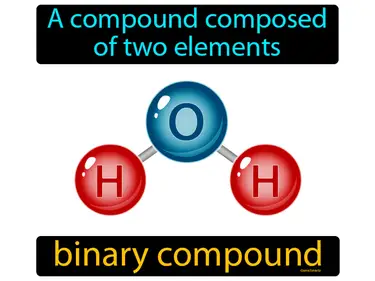
A compound composed of two elements. Binary compound. A binary compound is a chemical substance made of exactly two different elements.
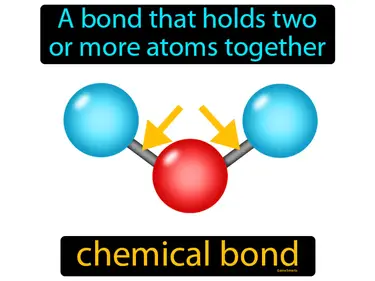
bond energy
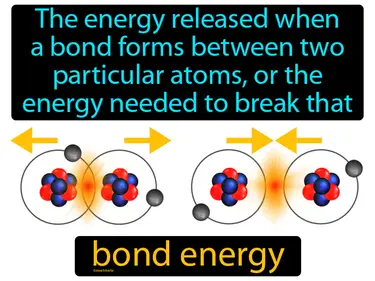
The energy released when a bond forms between two particular atoms, or the energy needed to break that bond. Bond energy. Bond energy is a measure of the strength of a chemical bond between atoms.
cation
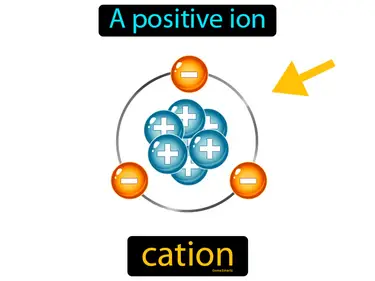
A positive ion. Cation. A cation is an atom or molecule that has lost one or more electrons, giving it a positive charge.
crystal lattice
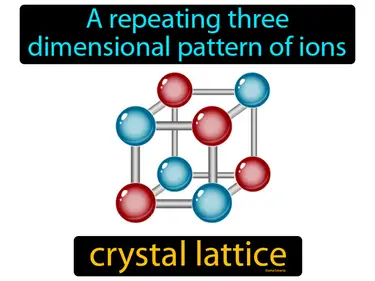
a repeating three dimensional pattern of ions. crystal lattice. It is a structure where ions are arranged in a regular, repeating pattern in three dimensions, forming a solid.
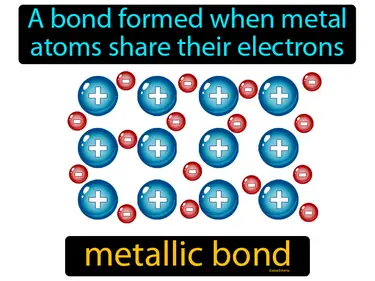
electron sea model
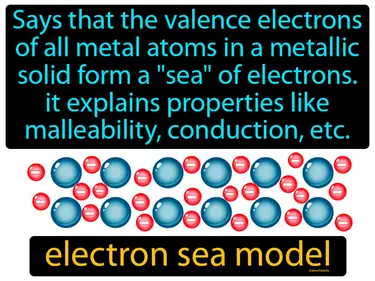
"Says that the valence electrons of all metal atoms in a metallic solid form a 'sea' of electrons. It explains properties like malleability, conduction, etc., electron sea model." The electron sea model describes how electrons flow freely around metal atoms, allowing them to conduct electricity and be shaped easily.
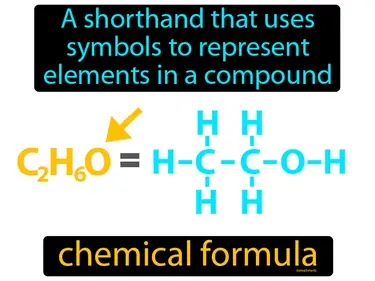
formula unit

The lowest whole-number ratio of ions in an ionic compound. Formula unit. A formula unit is the simplest ratio of ions represented in an ionic compound.
hydrate
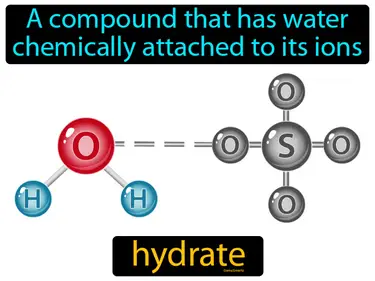
A compound that has water chemically attached to its ions. Hydrate. In simple terms, a hydrate is a substance that includes water molecules bonded to another compound or element.
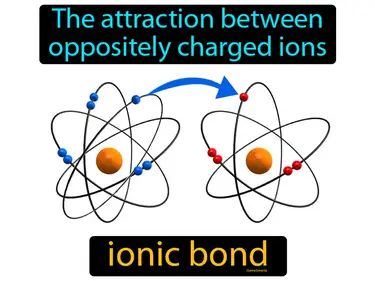
ionic compound
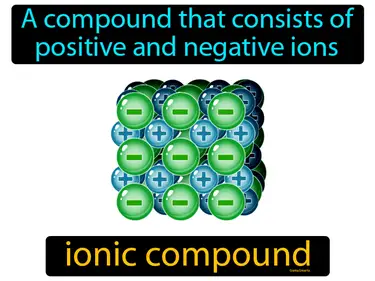
A compound that consists of positive and negative ions. Ionic compound. An ionic compound is a chemical substance made of charged particles that are held together by strong electrostatic forces.
lattice energy
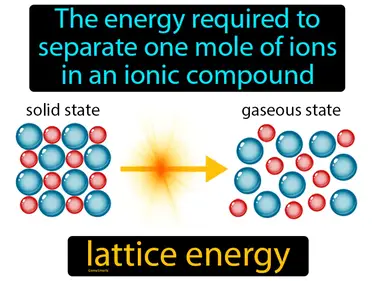
The energy required to separate one mole of ions in an ionic compound. Lattice energy. Lattice energy is the energy needed to break the bonds between ions in a solid ionic compound.
monatomic ion
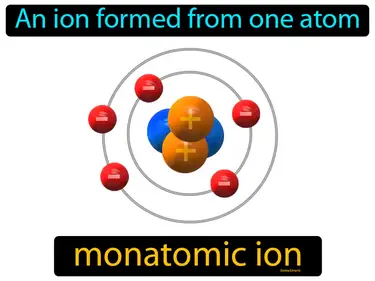
An ion formed from one atom. Monatomic ion. A monatomic ion is an atom that has gained or lost electrons, giving it a positive or negative charge.
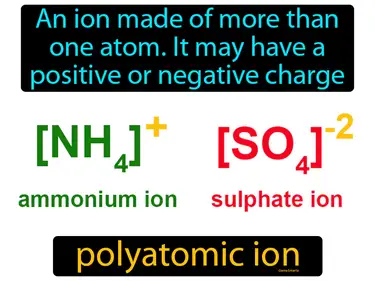
oxyanion
A polyatomic ion that contains oxygen. Oxyanion. An oxyanion is a negatively charged molecule made of oxygen and at least one other element.
triple bond
A chemical bond formed when two atoms share three pairs of electrons. Triple bond. A triple bond is a strong connection where two atoms share three pairs of electrons, making the bond very strong.