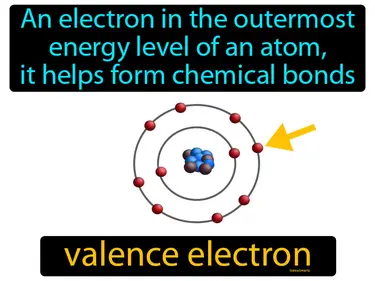
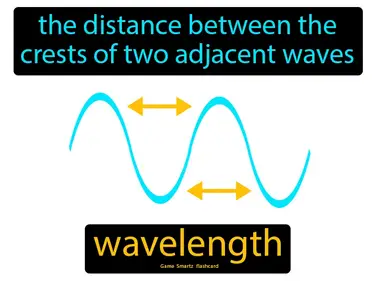
Electrons in Atoms
Science
atomic emission spectrum
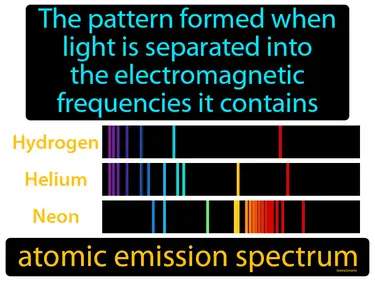
The pattern formed when light is separated into the electromagnetic frequencies it contains. Atomic emission spectrum. The atomic emission spectrum is a set of bright lines seen when elements emit light at specific wavelengths as electrons jump between energy levels.
atomic orbital
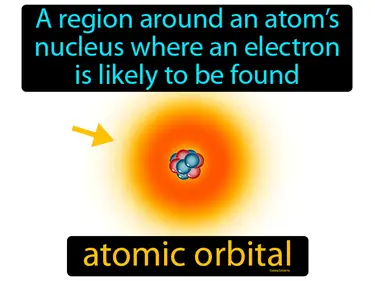
A region around an atoms nucleus where an electron is likely to be found. Atomic orbital. An atomic orbital is a region in an atom where there is a high chance of finding an electron.
aufbau principle
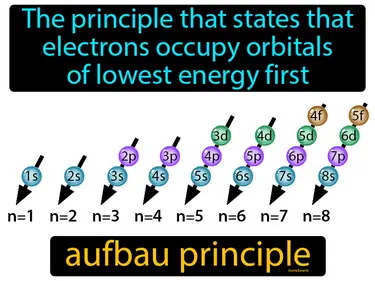
The principle that states that electrons occupy orbitals of lowest energy first. Aufbau principle. The aufbau principle explains how electrons fill the lowest energy levels before moving to higher ones in an atom.
electromagnetic radiation
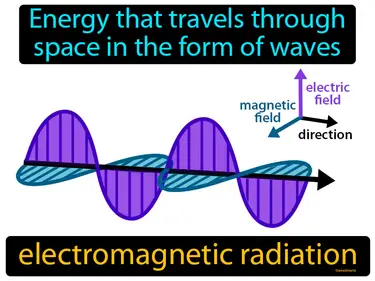
Energy that travels through space in the form of waves. Electromagnetic radiation. It's the way energy moves through space, like light or radio waves.
electron configuration
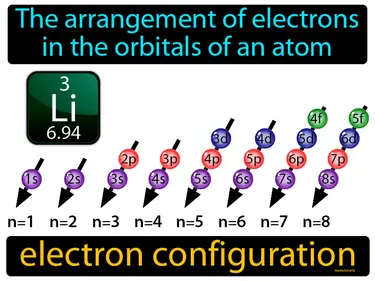
The arrangement of electrons in the orbitals of an atom. Electron configuration. It describes how electrons are distributed in an atoms orbitals, which determines the atom's chemical properties and behavior.
excited state

The state in which an atom has more energy than it does in its ground state. Excited state. An excited state is when an atom's electrons have absorbed energy and moved to higher energy levels.
ground state
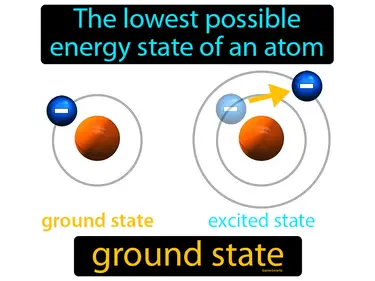
The lowest possible energy state of an atom ground state. Ground state is when an atom's electrons are in their normal, lowest energy positions.
Heisenberg uncertainty principle
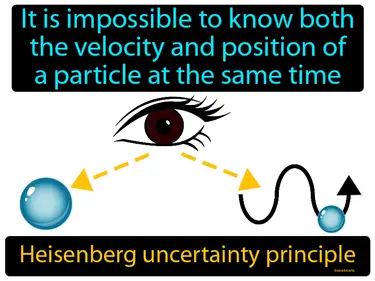
It is impossible to know both the velocity and position of a particle at the same time. Heisenberg uncertainty principle. This principle states that there are limits to how precisely we can measure both the position and momentum of a particle simultaneously.
Hunds rule
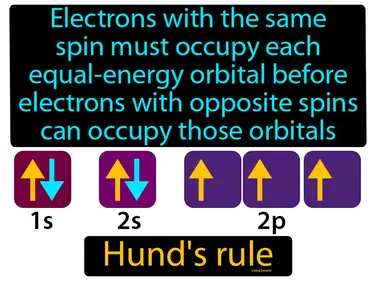
Electrons with the same spin must occupy each equal-energy orbital before electrons with opposite spins can occupy those orbitals. Hund's rule. Hund's rule states that electrons will fill empty orbitals of the same energy with their spins aligned before pairing up.
Pauli exclusion principle
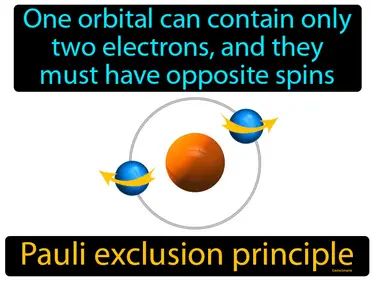
One orbital can contain only two electrons, and they must have opposite spins. Pauli exclusion principle. This principle states that no two electrons in an atom can have the same set of quantum numbers, essentially meaning they can't be in the exact same state.
photoelectric effect
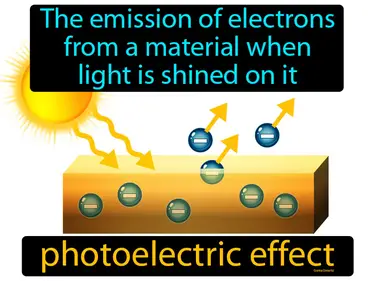
The emission of electrons from a material when light is shined on it. Photoelectric effect. When light hits a material, it can knock out electrons, causing an electric current.
photon
A particle or packet of light energy. Photon. A photon is a tiny particle that carries light and energy through space.
principal energy level
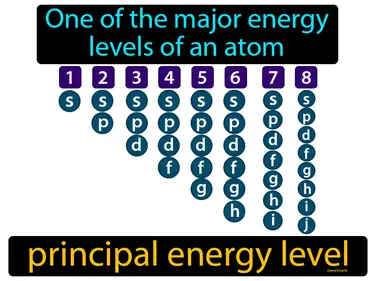
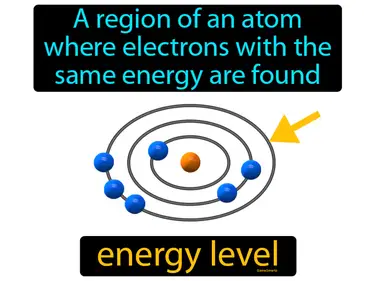
One of the major energy levels of an atom principal energy level. A principal energy level is the region around an atom's nucleus where electrons are likely to be found, with each level indicating a different distance from the nucleus and energy.
principal quantum number
Number indicating the relative sizes and energies of atomic orbitals. It is assigned by the quantum mechanical model. Principal quantum number. It is a number that tells us how far an electron is likely to be from the nucleus of an atom.
quantum

The minimum amount of energy that can be absorbed or emitted by an atom. Quantum. In simple terms, quantum refers to the smallest possible discrete unit of any physical property, like energy.
quantum mechanical model
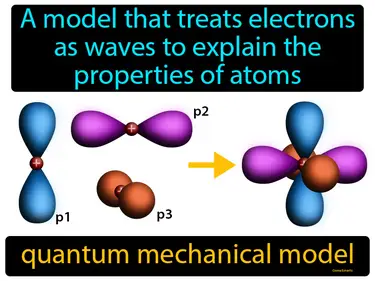
A model that treats electrons as waves to explain the properties of atoms. Quantum mechanical model. The quantum mechanical model describes how tiny particles, like electrons, behave in unpredictable ways that can't be explained by classical physics alone.
quantum number
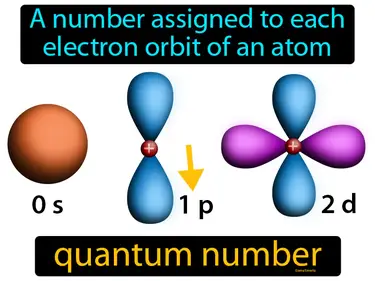
A number assigned to each electron orbit of an atom. Quantum number. It's like a unique address for an electron, helping us describe its position and energy in an atom.
sublevel
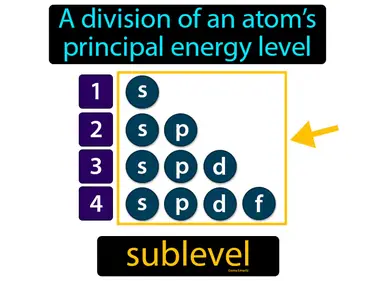
A division of an atoms principal energy level. Sublevel. A sublevel is a specific region within an energy level of an atom where electrons can be found.