Stoichiometry
Science
actual yield
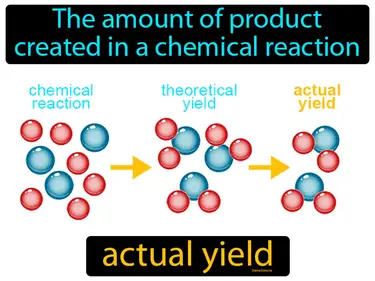
The amount of product created in a chemical reaction. Actual yield. Actual yield is the measured amount of a product obtained from a chemical reaction.
excess reactant
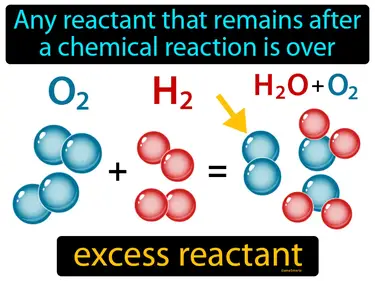
Any reactant that remains after a chemical reaction is over. Excess reactant. The excess reactant is the substance that is left over when a reaction has finished because there was more of it than needed to completely react with the other substances.
limiting reactant
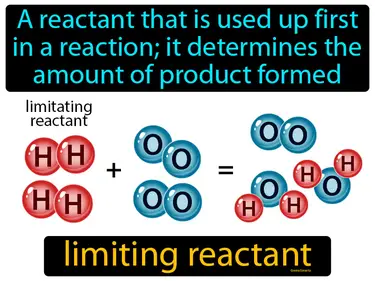
A reactant that is used up first in a reaction it determines the amount of product formed. Limiting reactant. The limiting reactant is the substance in a chemical reaction that runs out first, stopping the reaction and determining how much product is made.
mole ratio
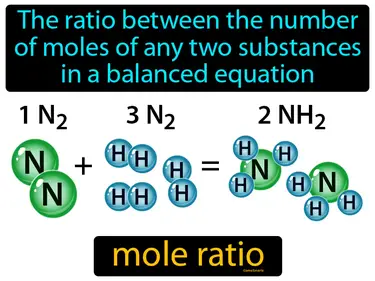
The ratio between the number of moles of any two substances in a balanced equation is called the mole ratio. In simple terms, a mole ratio tells you how many units of one substance react or are produced with units of another in a chemical reaction.
percent yield
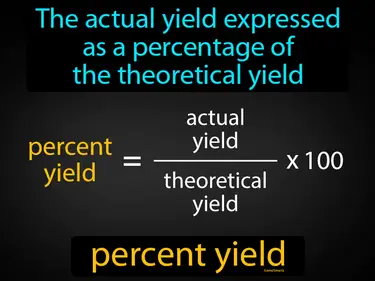
The actual yield expressed as a percentage of the theoretical yield. Percent yield. In Science, percent yield shows how efficient a chemical reaction is by comparing the actual amount of product to the maximum possible amount.
stoichiometry
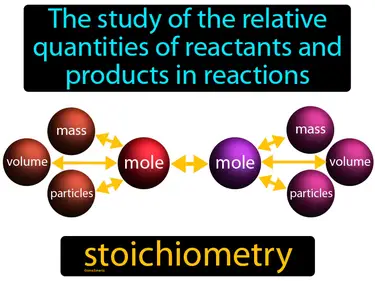
The study of the relative quantities of reactants and products in reactions. Stoichiometry. It is the calculation of the amounts of substances involved in chemical reactions.
theoretical yield
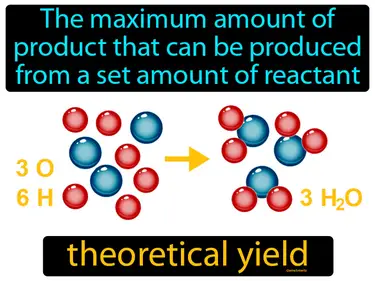
The maximum amount of product that can be produced from a set amount of reactant is called the theoretical yield. Theoretical yield is the calculated amount of product expected from a chemical reaction based on the reactants used, assuming everything goes perfectly.