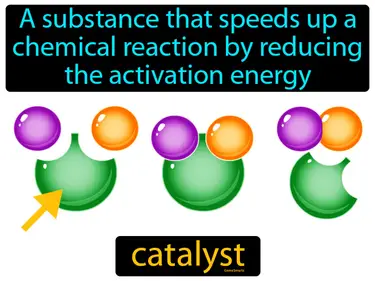
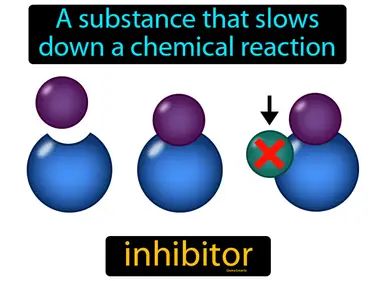
Reaction Rates
Science
activated complex
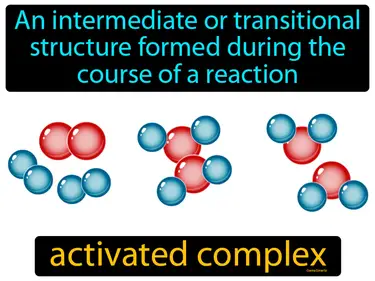
An intermediate or transitional structure formed during the course of a reaction. Activated complex. An activated complex is a temporary and unstable arrangement of atoms that forms at the peak of a chemical reaction's energy barrier.
collision theory
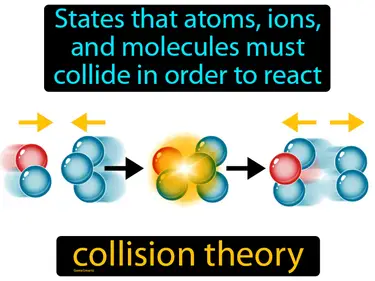
States that atoms, ions, and molecules must collide in order to react. Collision theory. In simple terms, collision theory explains that chemical reactions occur when particles crash into each other with enough energy and the right orientation.
heterogeneous catalyst
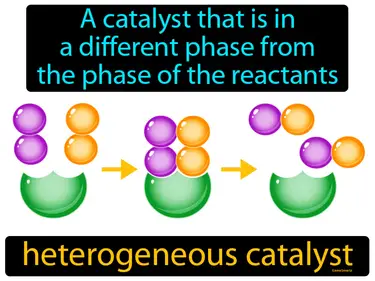
A catalyst that is in a different phase from the phase of the reactants. Heterogeneous catalyst. A heterogeneous catalyst speeds up a chemical reaction while being in a different state, like a solid catalyst working with gas reactants.
heterogeneous reaction
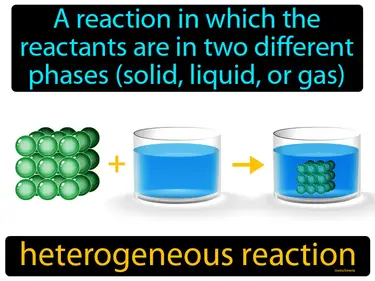
A reaction in which the reactants are in two different phases solid, liquid, or gas. Heterogeneous reaction. A heterogeneous reaction is when substances in different forms, like a solid and a liquid, react with each other.
homogeneous catalyst
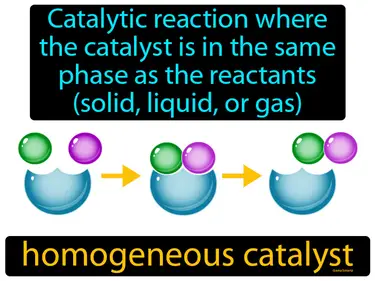
Catalytic reaction where the catalyst is in the same phase as the reactants, solid, liquid, or gas. Homogeneous catalyst. A homogeneous catalyst is a substance that speeds up a chemical reaction while being in the same physical state as the reactants.
homogeneous reaction
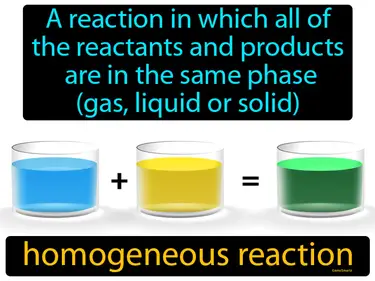
A reaction in which all of the reactants and products are in the same phase gas, liquid, or solid is called a homogeneous reaction. A homogeneous reaction occurs when substances mix uniformly in a single phase, like gases or liquids reacting together.
intermediate
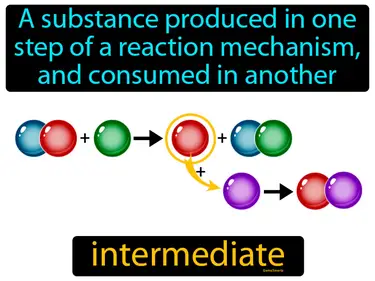
A substance produced in one step of a reaction mechanism, and consumed in another, is called an intermediate. In Science, an intermediate is a temporary substance formed during the steps of a chemical reaction.
rate law
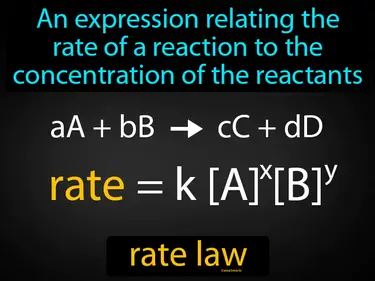
An expression relating the rate of a reaction to the concentration of the reactants. Rate law. Rate law is a mathematical equation that shows how the speed of a chemical reaction depends on the amount of reactants present.
rate-determining step
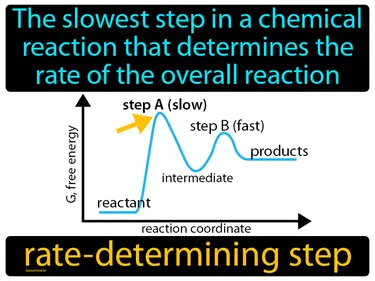
The slowest step in a chemical reaction that determines the rate of the overall reaction. Rate-determining step. It is the bottleneck step in a reaction that limits how fast the whole process can go.
reaction mechanism
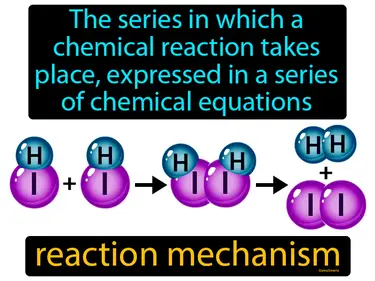
The series in which a chemical reaction takes place, expressed in a series of chemical equations. Reaction mechanism. A reaction mechanism explains the step-by-step sequence of events at the molecular level that leads to a chemical reaction.
reaction rate
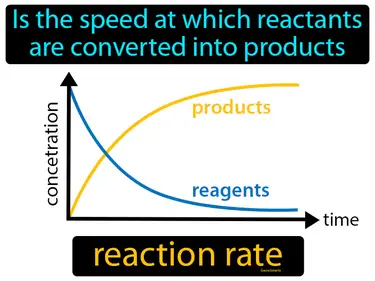
Is the speed at which reactants are converted into products. Reaction rate. It measures how quickly a chemical reaction occurs.
specific rate constant

A constant relating the concentrations of reactants to the rate of the reaction. Specific rate constant. It is a number that tells how fast a reaction proceeds under certain conditions.