Reduction
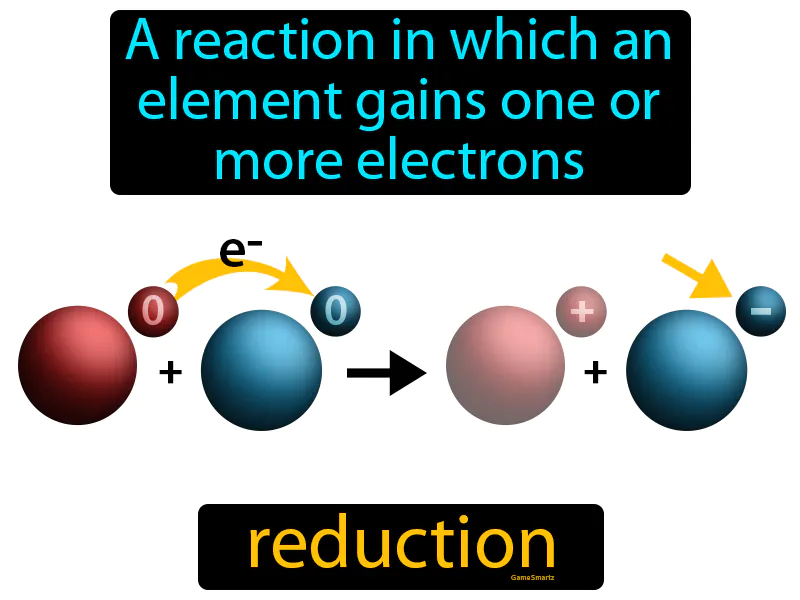
Imagine you're trying to collect rainwater in a series of buckets during a light drizzle. Just like each bucket gradually fills up with water, in a chemical reaction, an element gains one or more electrons to become "full" or stable. In this analogy, the buckets represent atoms or ions, the rainwater symbolizes the electrons, and the process of gaining water is akin to the reduction process in which the element becomes more stable by acquiring electrons.
Practice Version
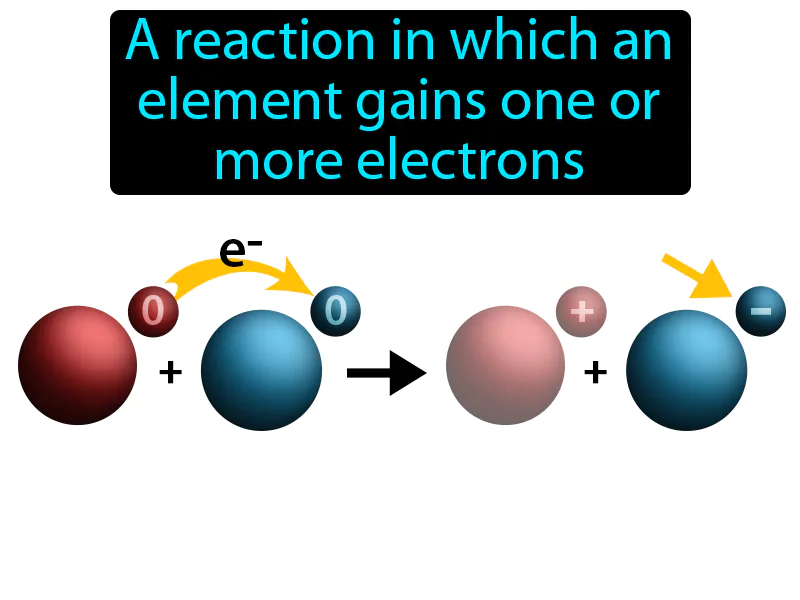
Reduction: A reaction in which an element gains one or more electrons. Reduction. In simple terms, reduction is the process where a substance gains electrons during a chemical reaction.