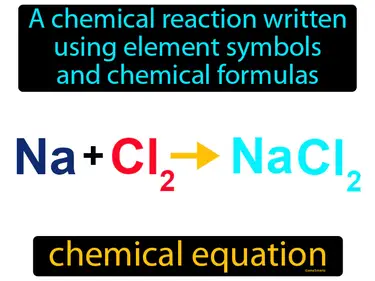
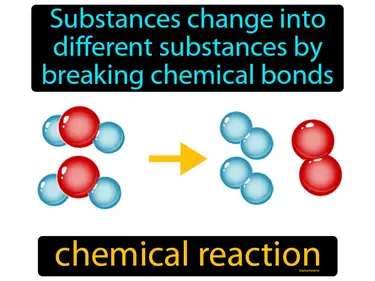
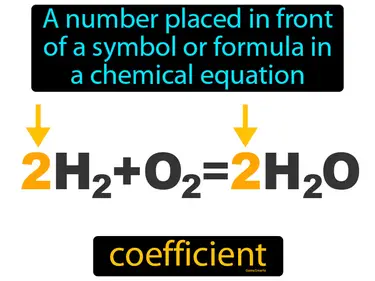
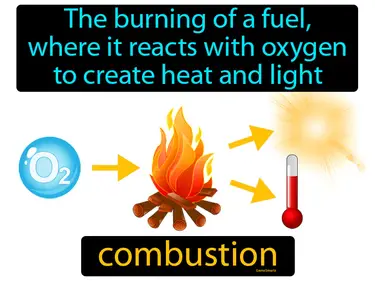
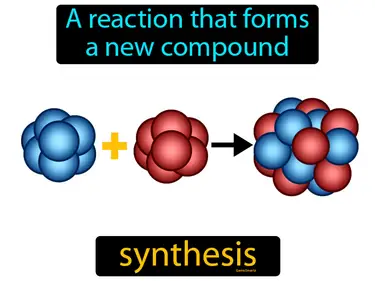
Chemical Reactions
Science
activity series
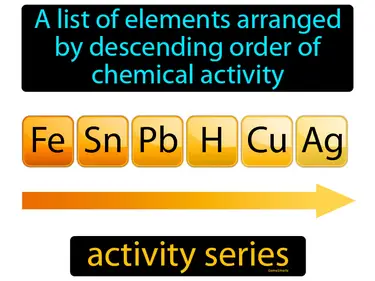
A list of elements arranged by descending order of chemical activity. Activity series. The activity series is a ranking of elements from most to least reactive, used to predict how they will behave in chemical reactions.
complete ionic equation
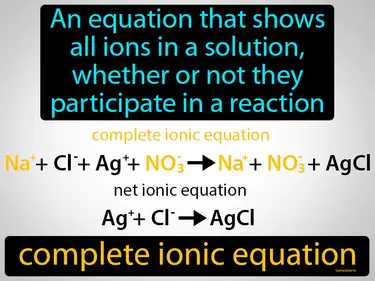
An equation that shows all ions in a solution, whether or not they participate in a reaction. Complete ionic equation. A complete ionic equation lists all the ions present in a chemical reaction in solution, showing which ions react and which remain unchanged.
net ionic equation
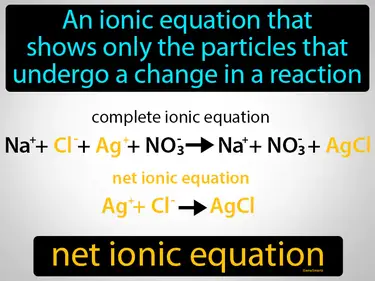
An ionic equation that shows only the particles that undergo a change in a reaction net ionic equation. A net ionic equation simplifies a chemical reaction by showing only the substances that actually participate in the reaction.
single-replacement reaction
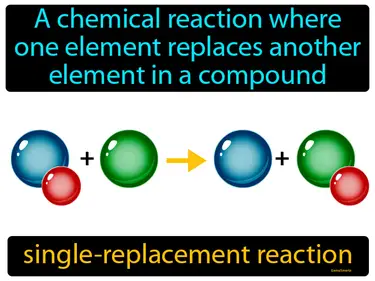
A chemical reaction where one element replaces another element in a compound. Single-replacement reaction. In this type of reaction, an element swaps places with another element in a compound.
spectator ion
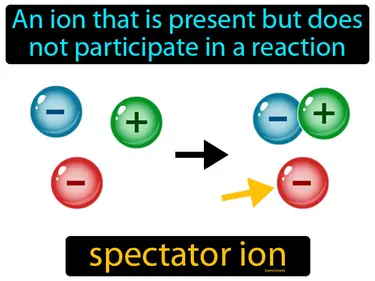
An ion that is present but does not participate in a reaction. Spectator ion. A spectator ion is an ion in a solution that exists in the same form on both the reactant and product sides of a chemical equation.