Pauli Exclusion Principle
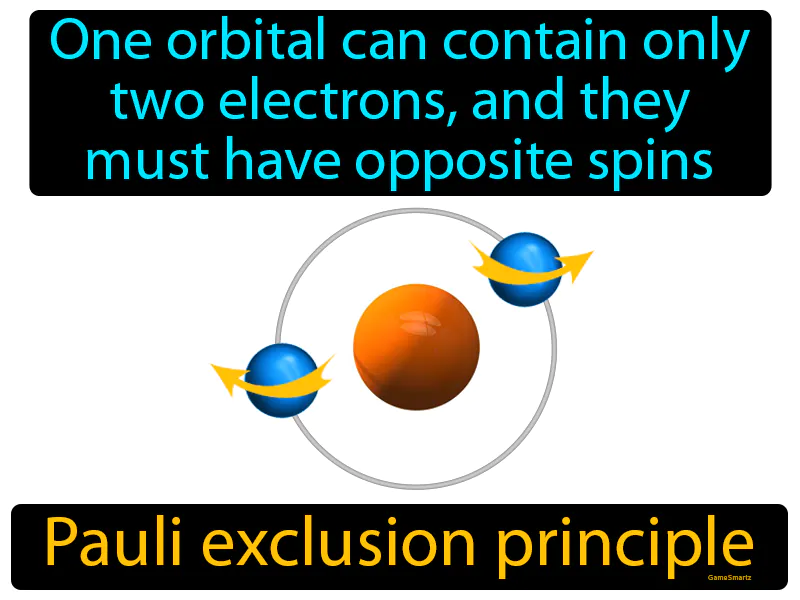
Imagine you're trying to find parking in a crowded lot, but each parking spot can only fit one compact car facing one direction and one facing the opposite direction. This is similar to the Pauli exclusion principle, where each orbital can hold only two electrons with opposite spins. Just as a parking spot accommodates only two cars positioned in opposite directions, an orbital can only hold two electrons that "spin" in opposite directions to maintain balance and order.
Practice Version
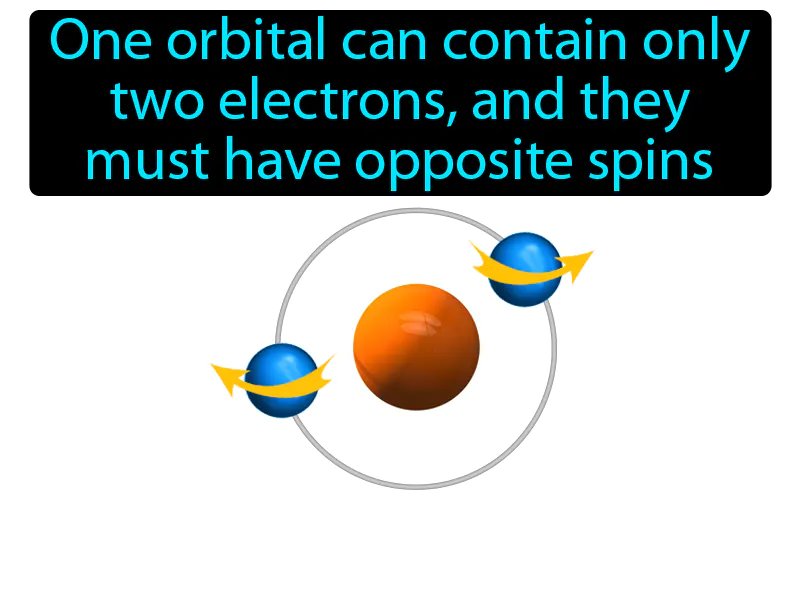
Pauli Exclusion Principle: One orbital can contain only two electrons, and they must have opposite spins. Pauli exclusion principle. This principle states that no two electrons in an atom can have the same set of quantum numbers, essentially meaning they can't be in the exact same state.