Hydrate
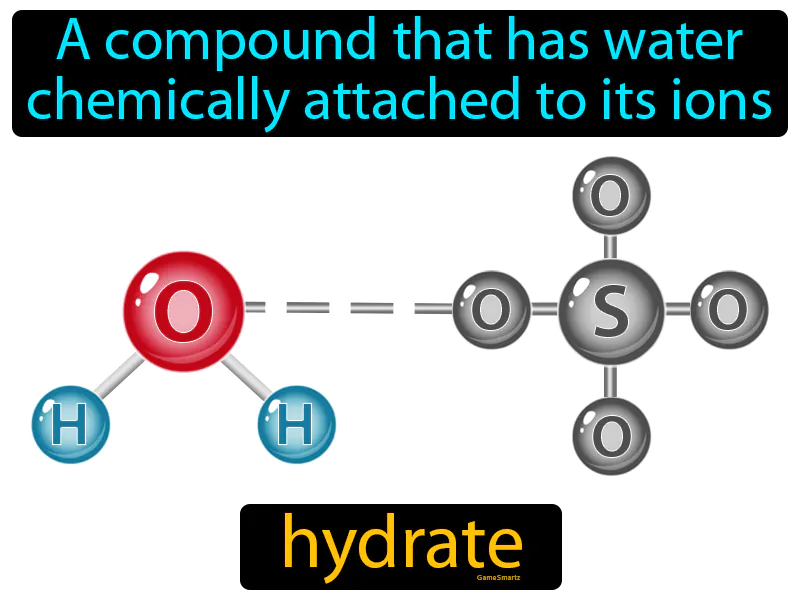
Imagine trying to carry multiple grocery bags in one hand while your other hand is occupied with your phone. This juggling act is similar to how a hydrate functions, where water molecules are "holding hands" with the compound's ions. Just like your hand is busy balancing the weight of the bags while still holding onto the phone, in a hydrate, water molecules are chemically attached to the compound, stabilizing it while contributing to its overall structure and properties.
Practice Version
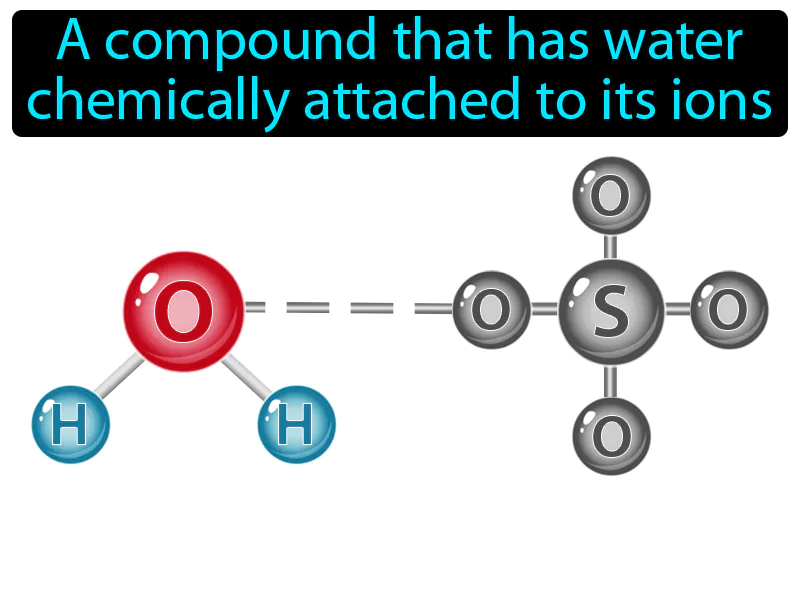
Hydrate: A compound that has water chemically attached to its ions. Hydrate. In simple terms, a hydrate is a substance that includes water molecules bonded to another compound or element.