Energy and the Ocean
Science
closed system
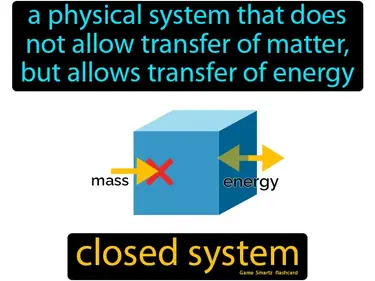
A physical system that does not allow transfer of matter, but allows transfer of energy. Closed system. In science, a closed system exchanges energy but not matter with its surroundings.
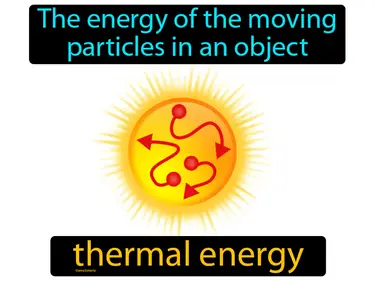
energy transformation
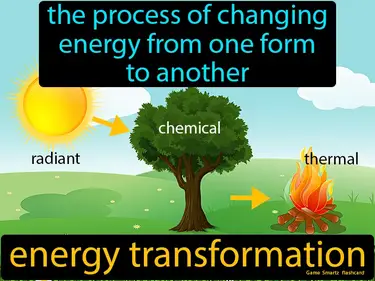
The process of changing energy from one form to another. Energy transformation. Energy transformation is when one type of energy changes into a different type, like when a battery converts chemical energy into electrical energy.
habitable

A potential to develop and maintain environments hospitable to life. Habitable. Habitable means a place that has the right conditions to support life.
heat capacity
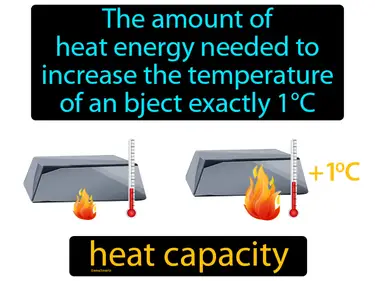
The amount of heat energy needed to increase the temperature of an object exactly 1C. heat capacity. Heat capacity is the amount of energy required to raise the temperature of an object by one degree Celsius.
isolated system
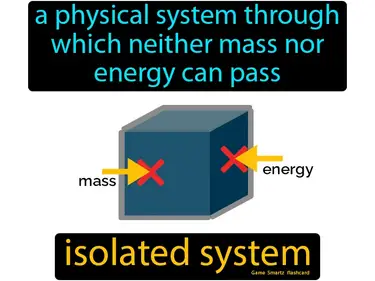
A physical system through which neither mass nor energy can pass. Isolated system. An isolated system is like a sealed box that nothing can enter or leave.
open system
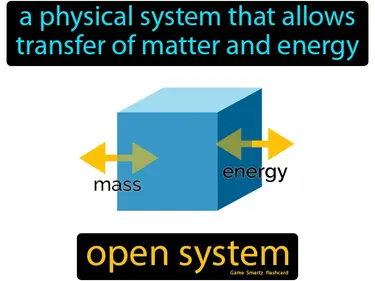
A physical system that allows transfer of matter and energy. Open system. An open system is one where matter and energy can enter and leave freely.
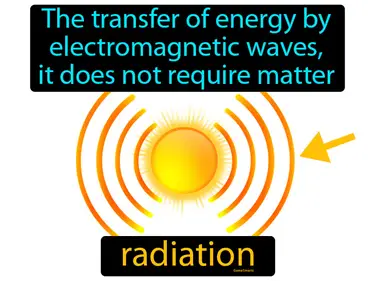
reradiate

When an object absorbs energy then emits it, reradiate. Reradiate is when an object sends out energy it has absorbed, often in the form of heat or light.
Second Law of Thermodynamics
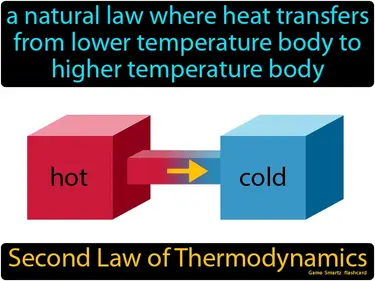
A natural law where heat transfers from lower temperature body to higher temperature body. Second Law of Thermodynamics. The Second Law of Thermodynamics states that energy naturally spreads out and becomes more disordered over time.
sink
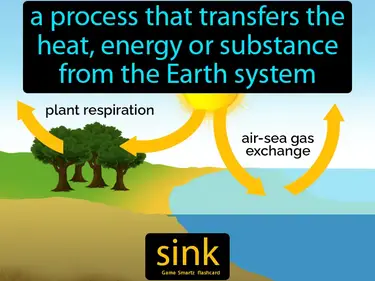
A process that transfers the heat, energy, or substance from the Earth system. A sink is a system that absorbs or stores energy or materials.
thermal energy transfer
The way the heat moves from one physical system to another. Thermal energy transfer. It's the process where heat flows from a warmer object to a cooler one until they reach the same temperature.
thermal equilibrium
A point where there is no net flow of thermal energy between two connected physical systems. Thermal equilibrium. It means two objects at the same temperature, so no heat flows between them.