Dipole
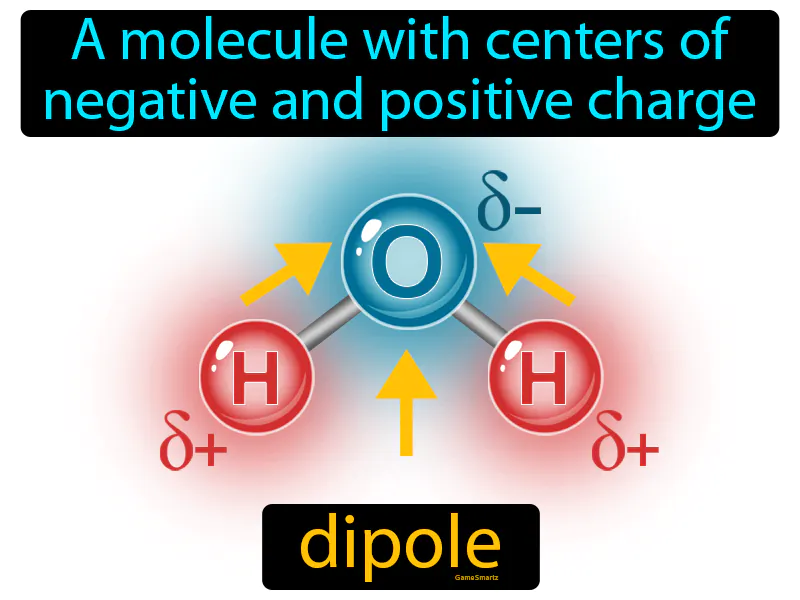
Imagine trying to balance a seesaw with one person sitting on each end. This scenario is similar to a dipole in a molecule, where you have centers of negative and positive charge acting like the two ends of the seesaw. Just as the seesaw has a pivot point in the middle, a molecule with a dipole has a separation between its positive and negative charges, creating a balance similar to how the seesaw balances with weight distributed on different ends.
Practice Version
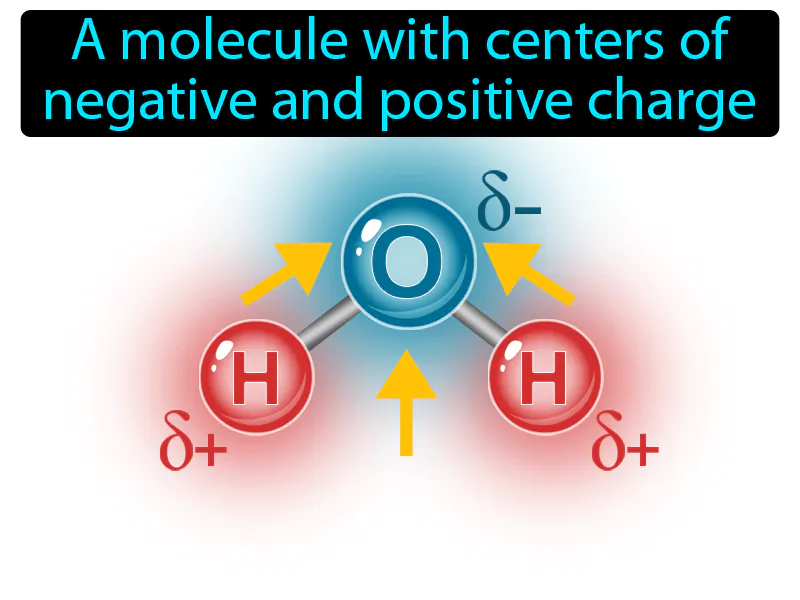
Dipole: A molecule with centers of negative and positive charge. Dipole. A dipole is when a molecule has a slight positive charge on one side and a slight negative charge on the other, like a tiny magnet.