Sigma Bond
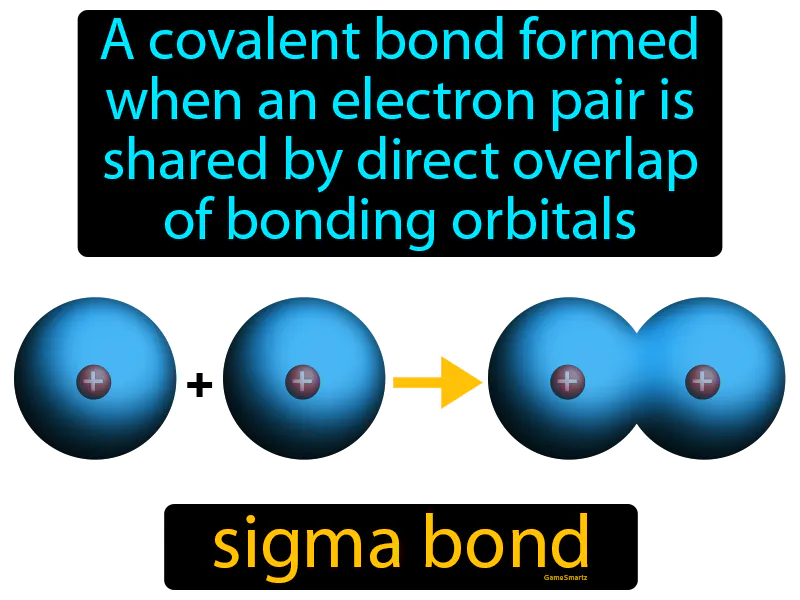
Imagine trying to fit two puzzle pieces together perfectly so they click into place. This is similar to how a sigma bond forms when two atoms' orbitals overlap directly, allowing them to share an electron pair seamlessly. Just as the puzzle pieces must align correctly to create a stable picture, the orbitals must align directly for the electron pair to be shared effectively, creating a stable bond between the atoms.
Practice Version
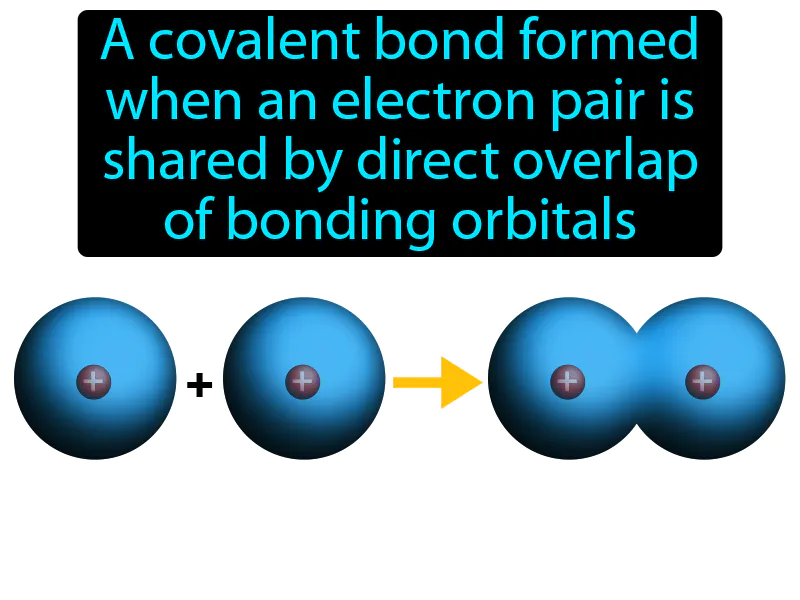
Sigma Bond: A covalent bond formed when an electron pair is shared by direct overlap of bonding orbitals. Sigma bond. A sigma bond is a type of covalent bond where two atoms share electrons in an overlapping manner, creating a strong bond.