Coordinate Covalent Bond
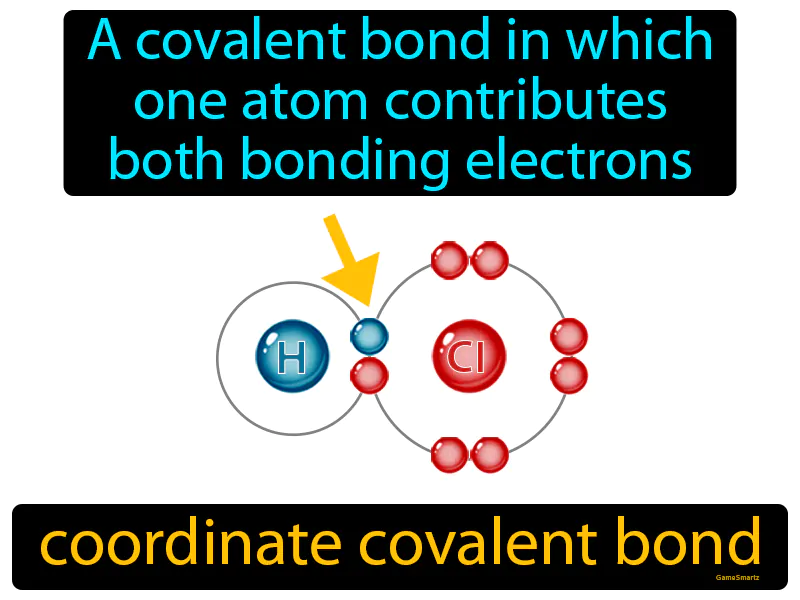
Imagine you're planning a potluck dinner, but one friend shows up without any food to share. This is similar to a coordinate covalent bond, where one atom provides both electrons for the bond because the other atom doesn't contribute any. In this analogy, your friend represents the atom lacking resources (electrons), while you, bringing extra food to share, symbolize the atom donating both electrons to form a stable bond, ensuring everyone enjoys the meal together.
Practice Version
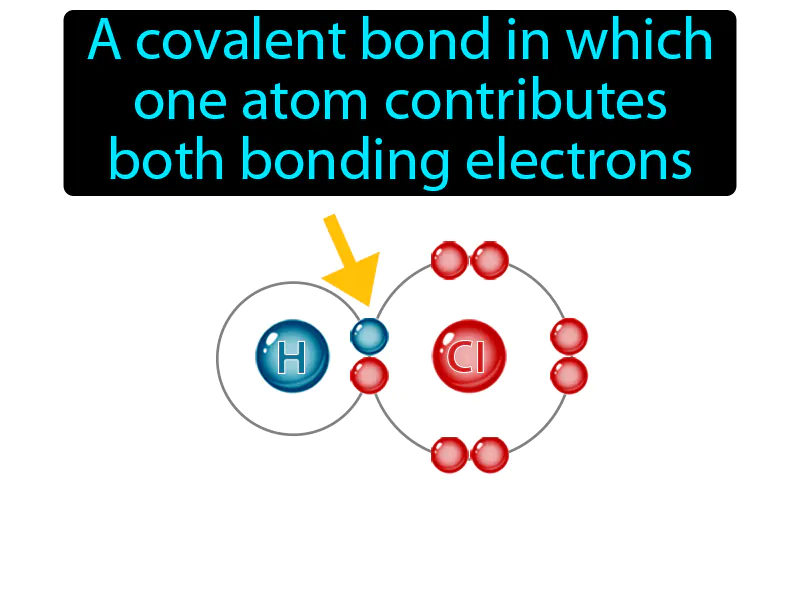
Coordinate Covalent Bond: A covalent bond in which one atom contributes both bonding electrons. Coordinate covalent bond. In a coordinate covalent bond, one atom provides both of the electrons needed to form the bond with another atom.