Triple Covalent Bond
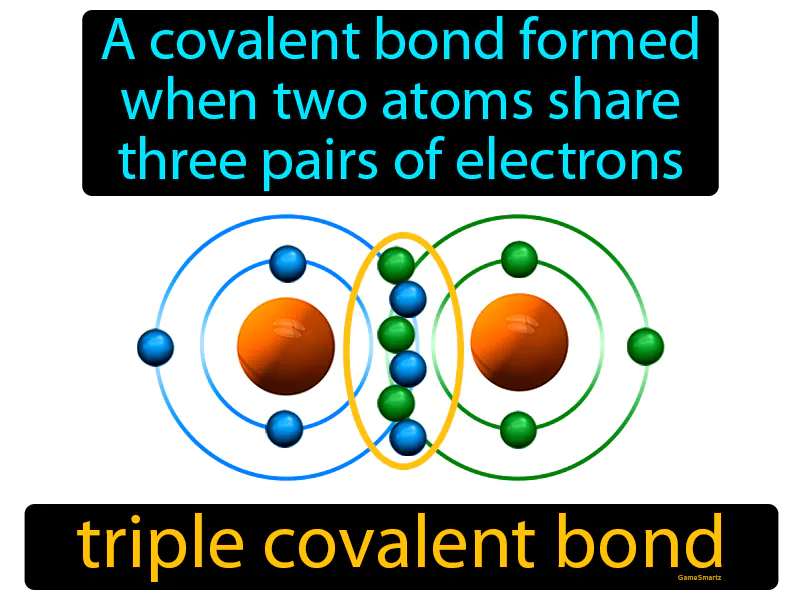
Imagine trying to hold three large beach balls between two people without letting any drop. This scenario is similar to a triple covalent bond, where two atoms "hold" or share three pairs of electrons tightly between them. Just as the two people must coordinate and work together to maintain balance and keep all three beach balls from falling, two atoms share three pairs of electrons to form a stable, strong connection, illustrating how they rely on each other to maintain stability.
Practice Version
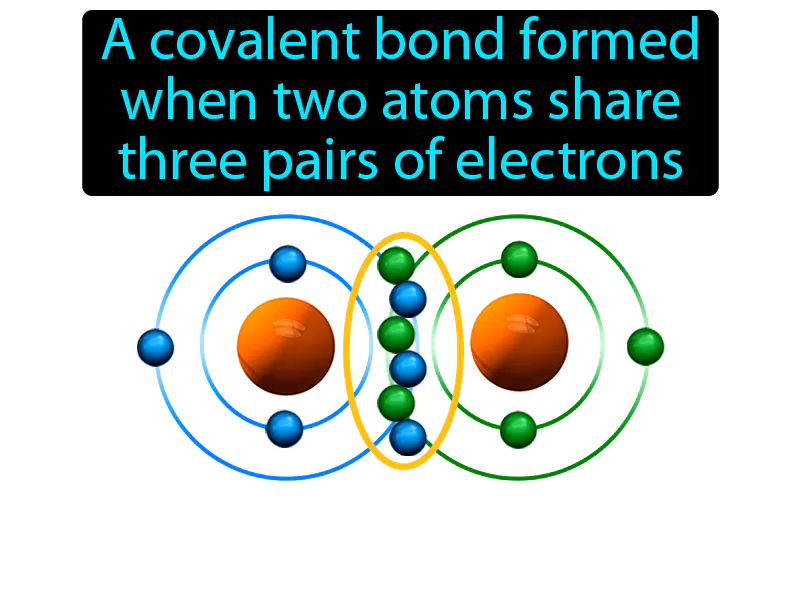
Triple Covalent Bond: A covalent bond formed when two atoms share three pairs of electrons. Triple covalent bond. In simple terms, a triple covalent bond is when two atoms share three pairs of electrons to stay connected.