Bronsted-Lowry Model
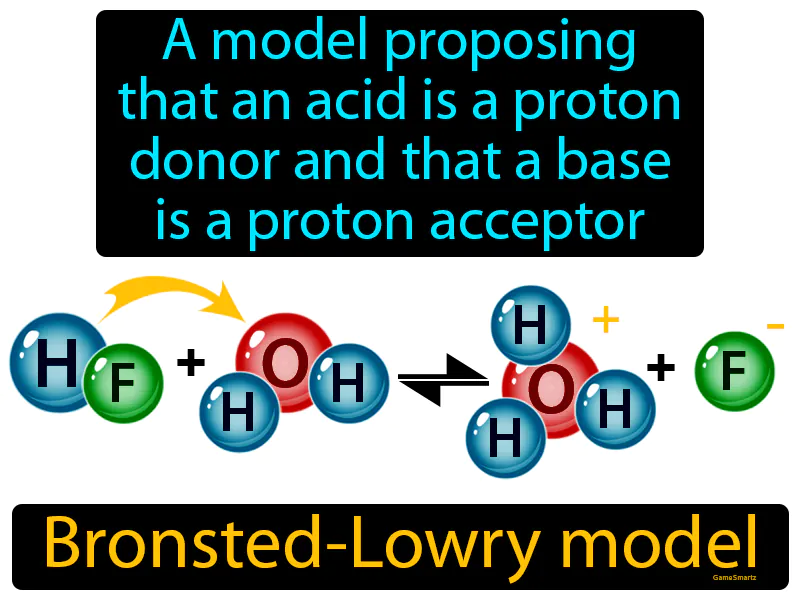
Imagine you’re at a dinner party and the host is offering cups of lemonade, but there aren't enough cups for everyone. This situation is similar to the Bronsted-Lowry model, where an acid is like the host giving away cups (protons), and a base is like a guest who accepts a cup. In this analogy, the host represents the acid, generously donating cups (protons) to the guests, while the guests represent the base, readily accepting the cups offered to them.
Practice Version
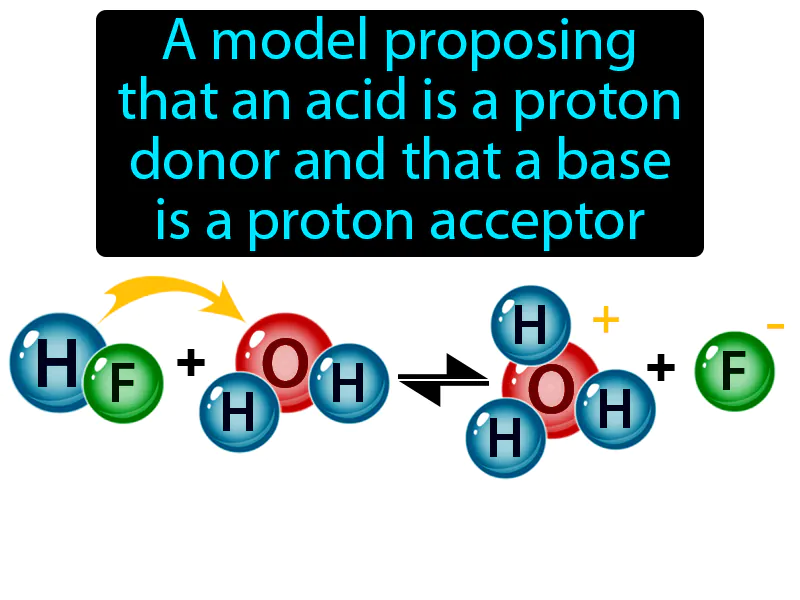
Bronsted-Lowry Model: A model proposing that an acid is a proton donor and that a base is a proton acceptor. Bronsted-Lowry model. The Bronsted-Lowry model explains acids and bases in terms of their ability to donate or accept protons, respectively.