Intermolecular Force
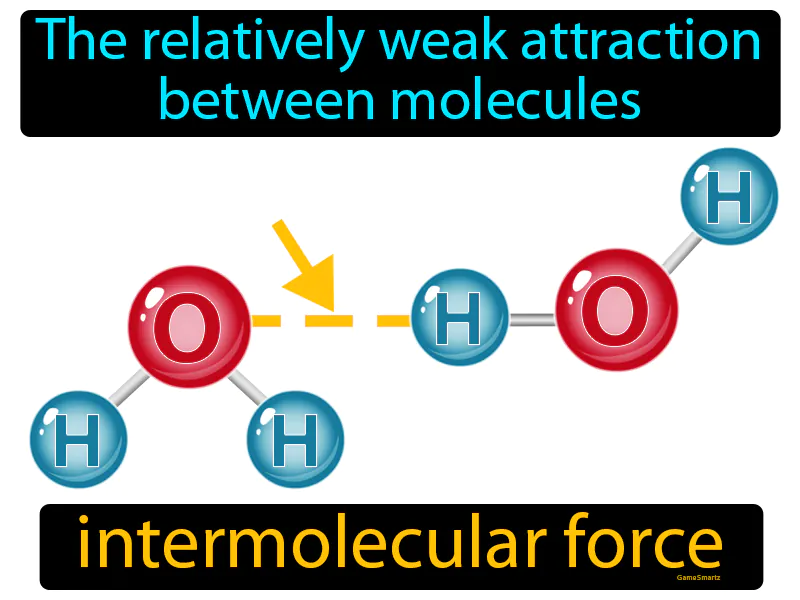
Imagine trying to keep a stack of papers neatly in place on a slightly windy day. Just as these papers are lightly held together by gravity and friction but can easily be disturbed by a gentle breeze, molecules within a substance are attracted to each other by relatively weak forces that can be disrupted by slight changes in conditions. The connection here is that the stack of papers represents molecules being held together; the wind represents external factors like temperature or pressure that can affect the weak attractions, just as a small breeze can scatter the papers.
Practice Version
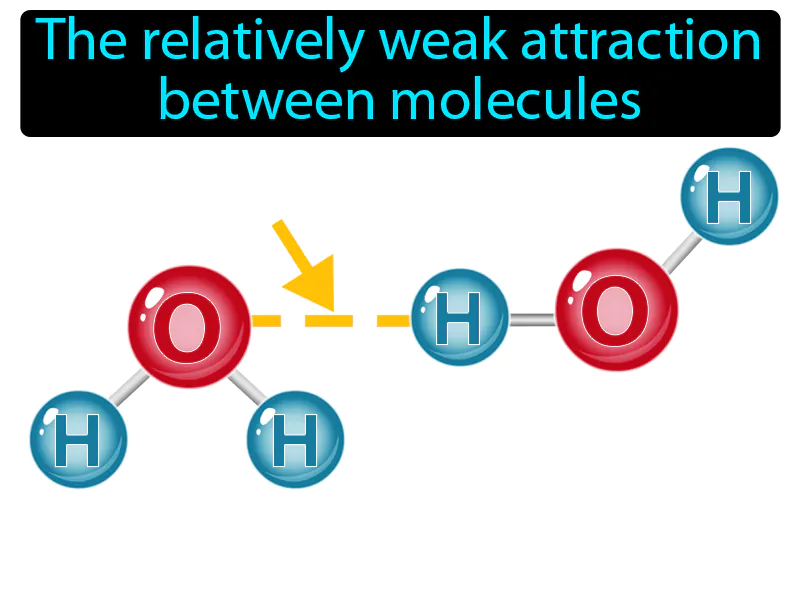
Intermolecular Force: The relatively weak attraction between molecules. Intermolecular force. Intermolecular force is the force that holds molecules close to each other, affecting their physical properties like boiling and melting points.