Kinetic-molecular Theory
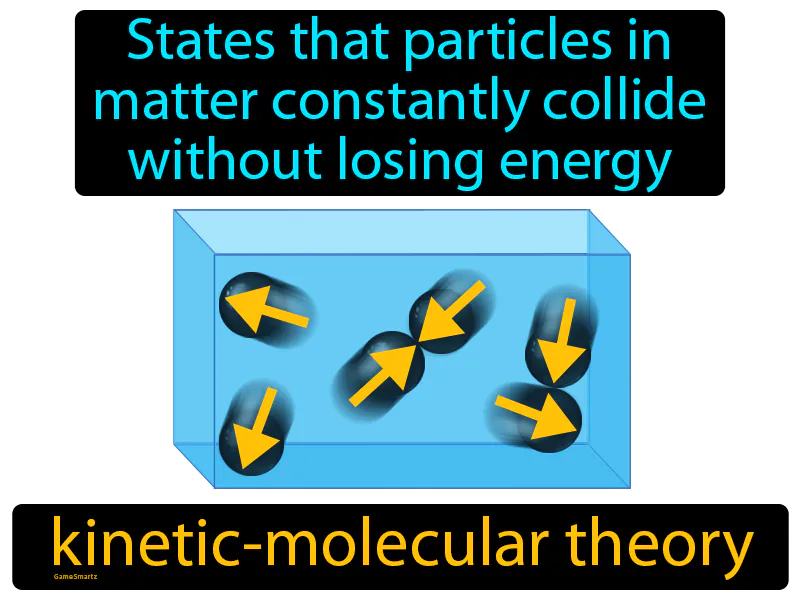
Imagine you're at a crowded party where everyone is moving around, chatting, and mingling without ever getting tired. Just like the guests at the party, particles in matter are constantly in motion, colliding with each other but never losing energy or slowing down. Each person at the party represents a particle, and their continuous movement and interactions mirror how particles behave according to the kinetic-molecular theory, maintaining their energy and momentum as they move through the space.
Practice Version
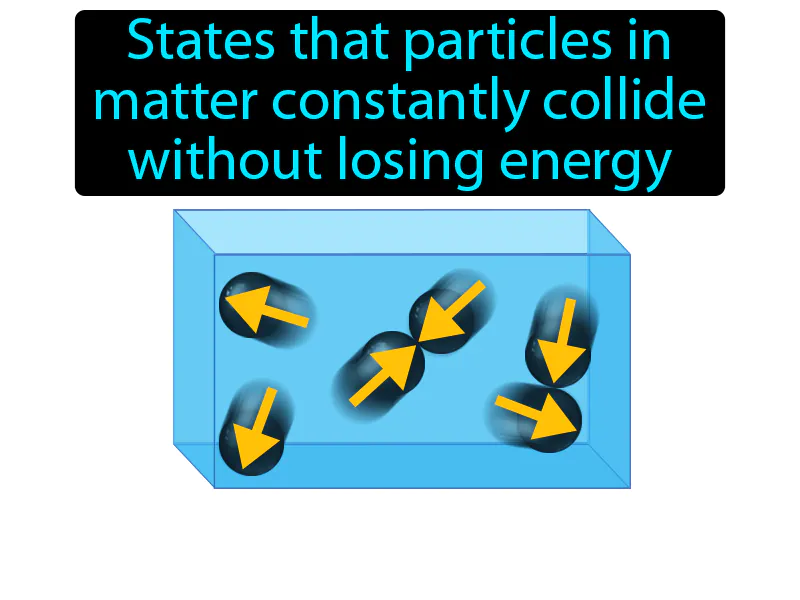
Kinetic-molecular Theory: States that particles in matter constantly collide without losing energy. Kinetic-molecular theory. It explains how the tiny particles in matter move around and interact, helping us understand things like gases and temperature.