Heat Of Vaporization
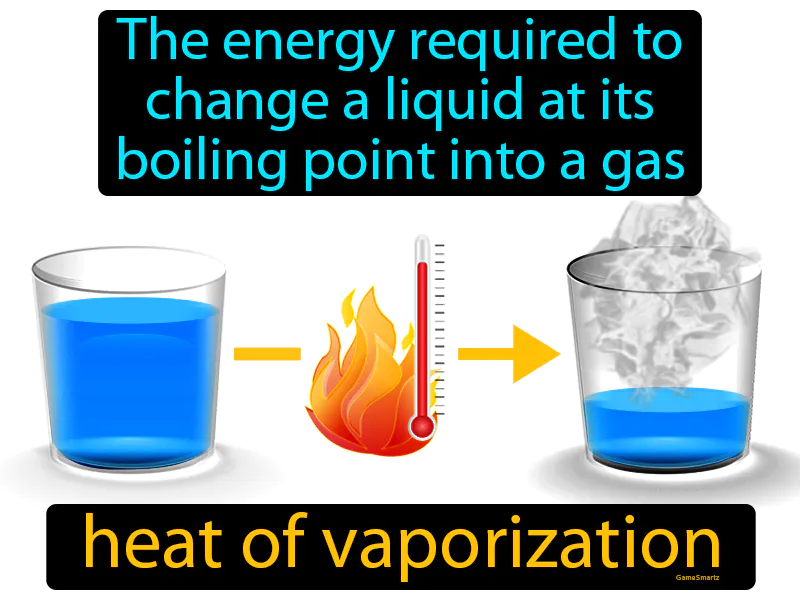
Imagine trying to wake up in the morning when you're feeling incredibly sleepy. This situation is similar to the heat of vaporization because just like you need a burst of energy or motivation to transition from a sleepy state to being fully awake and active, a liquid needs energy to transition from its liquid state to a gas at its boiling point. In this analogy, your sleepy state represents the liquid, the energy or motivation you need is the heat of vaporization, and becoming fully awake and active is akin to the liquid turning into a gas.
Practice Version
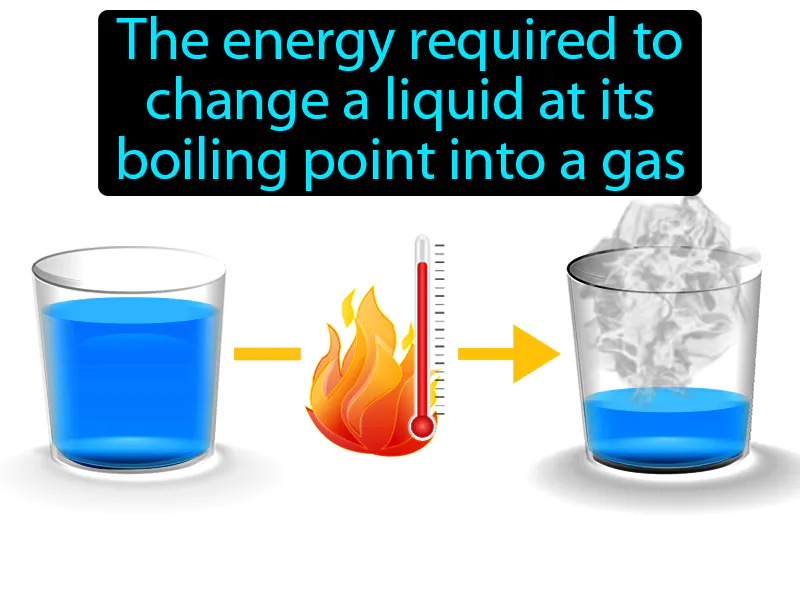
Heat Of Vaporization: The energy required to change a liquid at its boiling point into a gas. Heat of vaporization. Heat of vaporization is the amount of energy needed to turn a liquid into a gas at its boiling point without changing its temperature.