Equilibrium Position
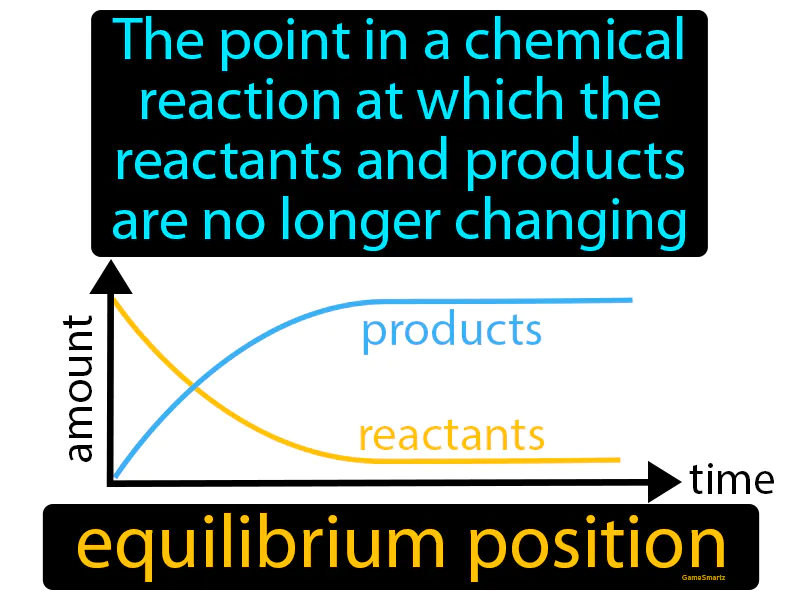
Imagine you're trying to balance two kids on a seesaw at the playground so that neither side is going up or down. This situation is similar to a chemical reaction reaching equilibrium, where the reactants and products are balanced and no longer changing in concentration. Just like finding the perfect position on the seesaw where the weight of each child keeps it level, in a chemical reaction, equilibrium is the point where the forward and reverse reactions occur at the same rate, maintaining a stable balance between reactants and products.
Practice Version
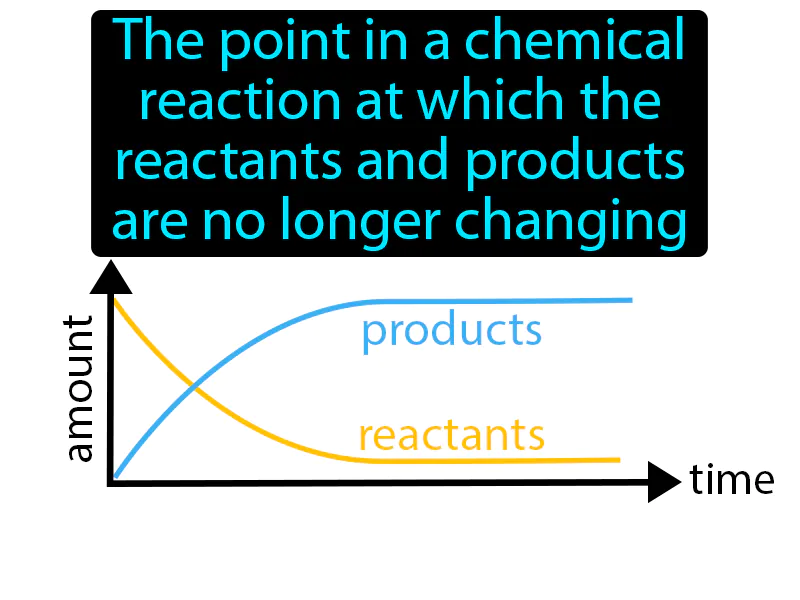
Equilibrium Position: The point in a chemical reaction at which the reactants and products are no longer changing equilibrium position. Equilibrium position is the state in a reaction where the amounts of reactants and products stay constant over time.