Law Of Chemical Equilibrium
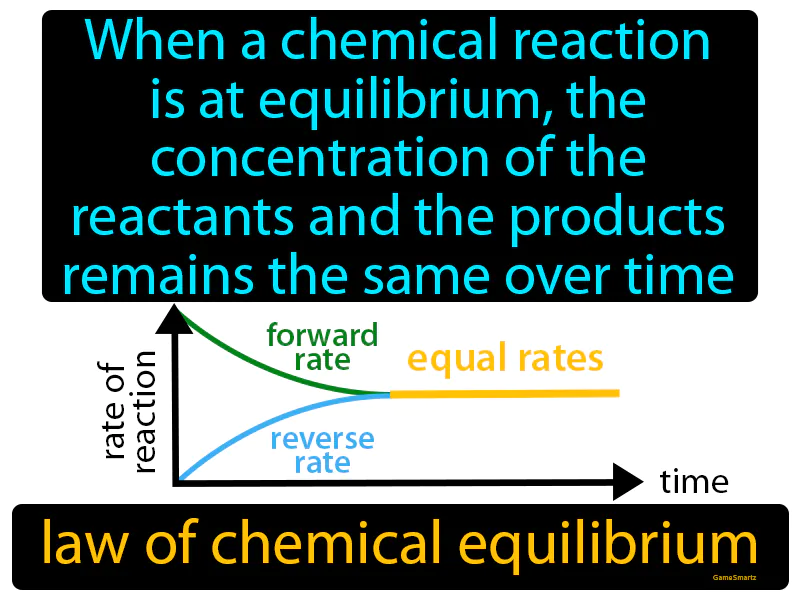
Imagine you're trying to balance two sides of a seesaw in a playground, where your friend is sitting on one side and you are on the other, shifting your weight to keep it level. This situation is similar to a chemical reaction at equilibrium, where the concentrations of reactants and products stay constant because both sides are perfectly balanced. Just as the seesaw doesn't tip over when both you and your friend adjust to maintain balance, a reaction at equilibrium doesn't change its concentrations because the forward and reverse reactions occur at equal rates, keeping everything steady and unchanged over time.
Practice Version
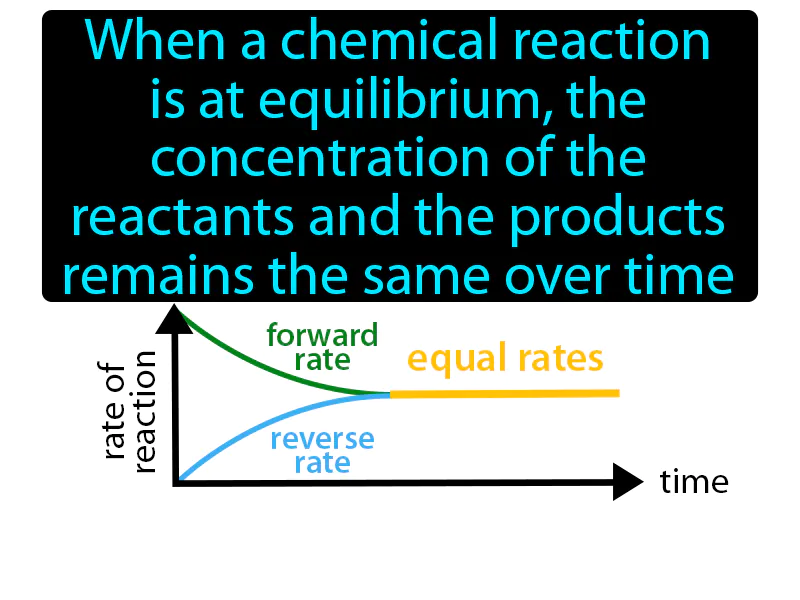
Law Of Chemical Equilibrium: When a chemical reaction is at equilibrium, the concentration of the reactants and the products remains the same over time. Law of chemical equilibrium. It means that at equilibrium, the rates of the forward and reverse reactions are equal, keeping concentrations constant.