Molarity
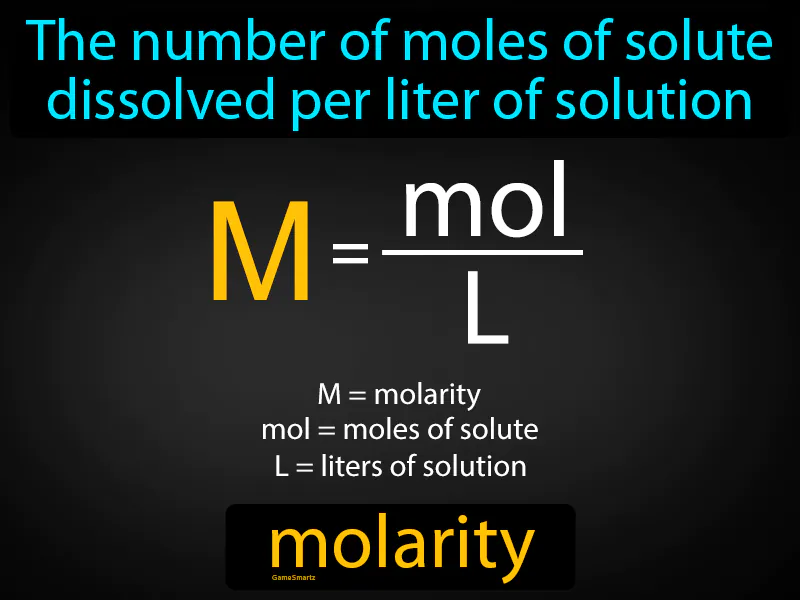
Imagine you're hosting a party and need to decide how many snacks to provide for every guest attending. This is similar to molarity, where instead of snacks and guests, you have the amount of solute and the volume of solution. Just as you determine the number of snacks per guest to ensure everyone is satisfied, molarity measures how much solute is present in a given volume of solution to ensure the desired concentration.
Practice Version
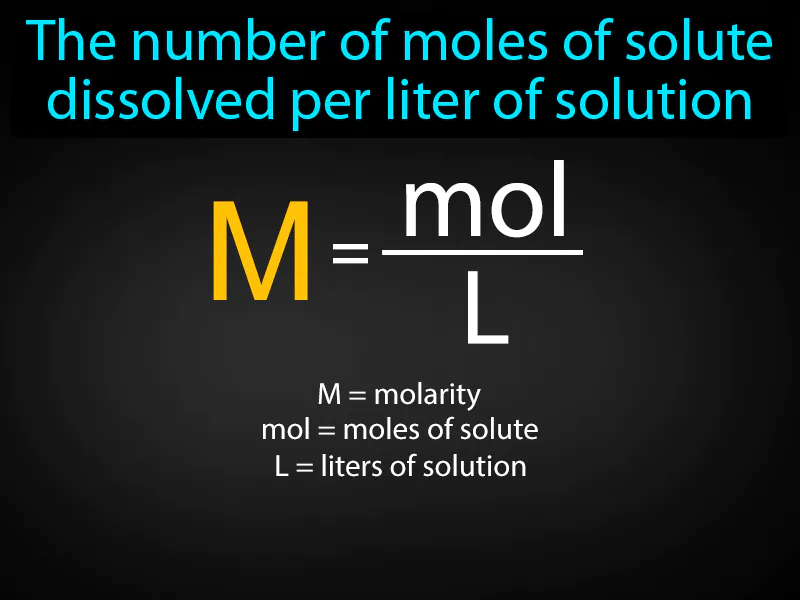
Molarity: The number of moles of solute dissolved per liter of solution. Molarity. Molarity is a measure of how concentrated a solution is, telling us how much solute is present in a specific volume of liquid.