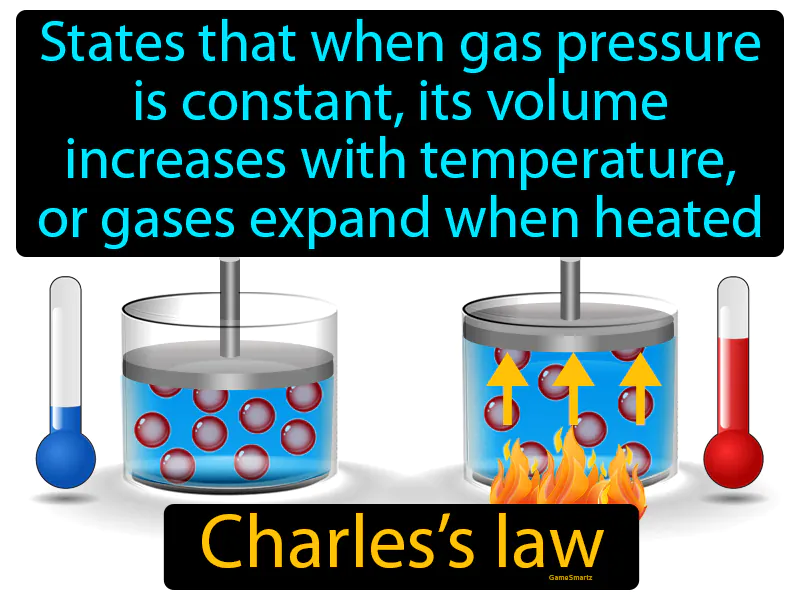
Charles Law
Charles law explained in an easy to understand way:
Imagine trying to fit into your favorite pair of jeans right after a big holiday meal. Just like your waistband feels tighter after eating, a gas will take up more space when heated. Both scenarios involve expansion: your post-meal belly expands like a gas does with rising temperatures, keeping pressure constant. This illustrates Charles's Law, where the 'fullness' of the gas (or your stomach) increases, leading to more volume when warmed up, even though the overall 'pressure' (the waistband or the gas environment) remains unchanged.
Practice Version
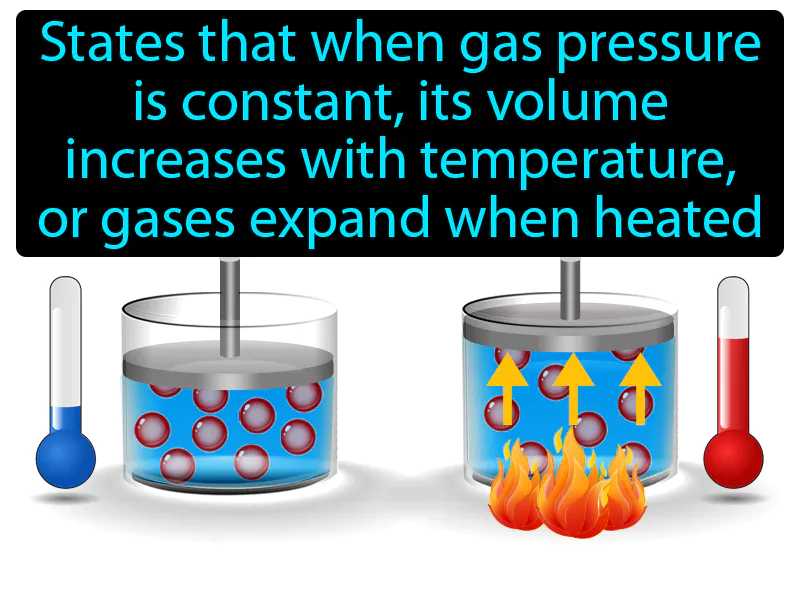
Charles Law: States that when gas pressure is constant, its volume increases with temperature, gases expand when heated. Charles's Law. It explains how increasing the temperature of a gas causes it to expand if the pressure remains unchanged.