Law Of Multiple Proportion
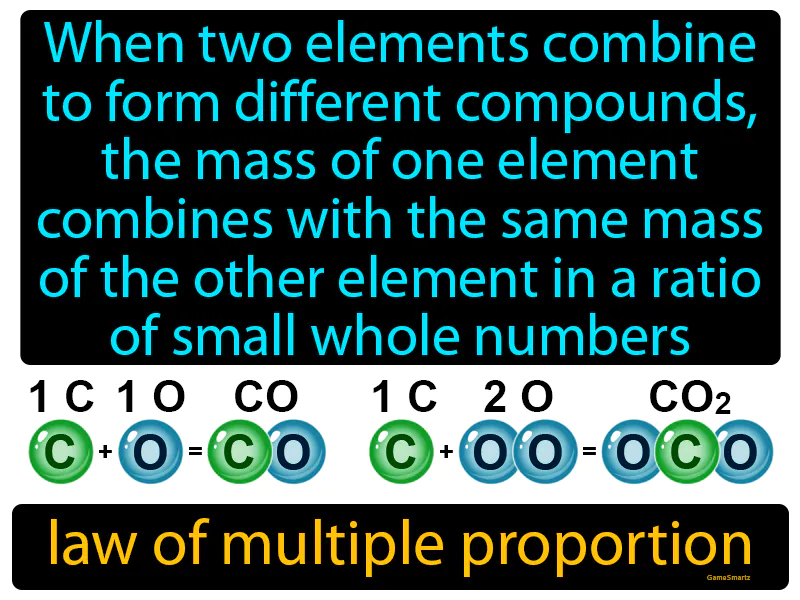
Imagine trying to make different types of sandwiches, where you have a fixed amount of bread, but you can use varying amounts of different fillings like cheese or ham. Just as the bread stays constant while you adjust the amount of fillings, elements combine in fixed ratios to form different compounds, where the mass of one element is constant, and the other varies. In this analogy, the bread represents the fixed mass of one element, while the different amounts of fillings represent how the varying masses of another element combine in small whole number ratios to form distinct sandwiches, or in scientific terms, different compounds.
Practice Version
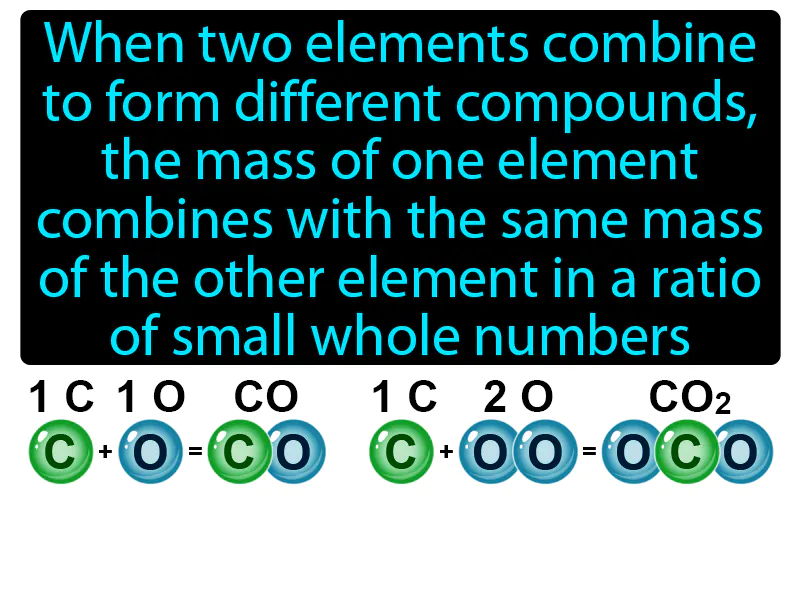
Law Of Multiple Proportion: When two elements combine to form different compounds, the mass of one element combines with the same mass of the other element in a ratio of small whole numbers. Law of multiple proportion. This law states that elements can combine in different ways to form different compounds, but the ratios of the masses of these compounds will be simple whole numbers.