Hydroxyl Group
Hydroxyl group explained in an easy to understand way:
Imagine you are trying to fit a specific key into a lock that only accepts keys with a certain shape. Just as a lock requires a key with a precise shape to open, the hydroxyl group (-OH) in alcohols is like that specific key that fits into chemical locks, allowing alcohol molecules to interact with other molecules in specific ways. In this analogy, the lock is the chemical reaction or interaction site, and the hydroxyl group acts as the key that enables the alcohol molecule to participate in these reactions, influencing properties such as solubility and reactivity.
Practice Version
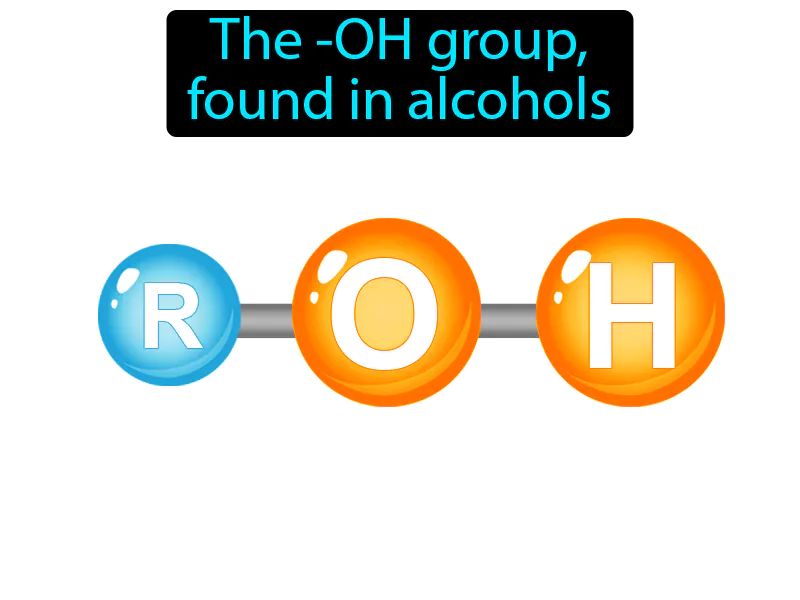
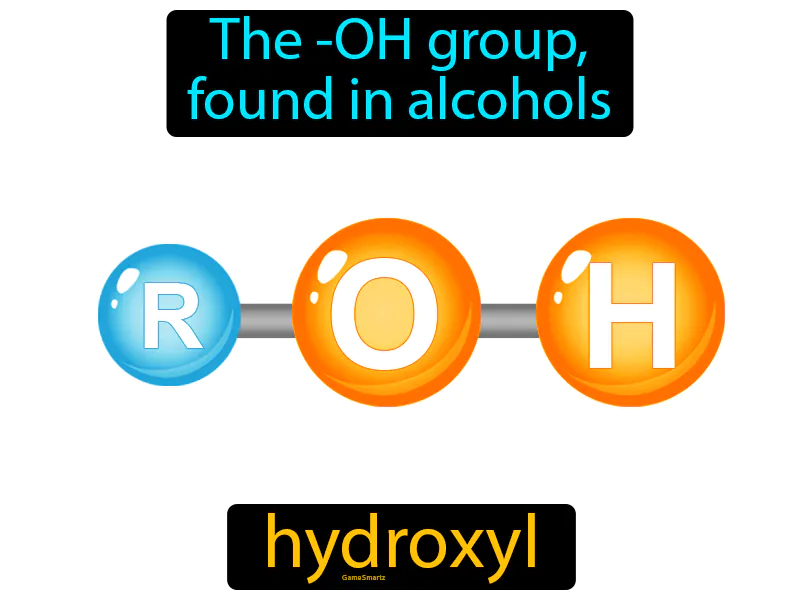
Hydroxyl Group: The -OH group found in alcohols. Hydroxyl group. The hydroxyl group is a pair of atoms consisting of an oxygen and a hydrogen that makes alcohols and other compounds reactive and soluble in water.