Anode
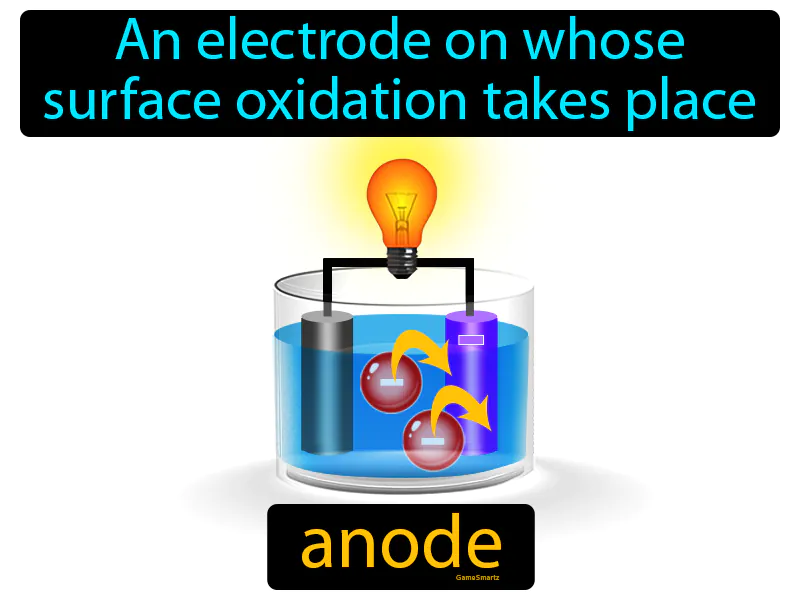
Imagine you're cooking and notice that the oil in your pan gradually turns darker as it heats and reacts with the food particles. Just like the oil changes due to its interaction with food, an anode is where oxidation occurs, meaning it loses electrons in a chemical reaction. In this analogy, the oil represents the anode, as both undergo a transformation through a process: the oil darkens with heat and food, while the anode loses electrons during oxidation.
Practice Version
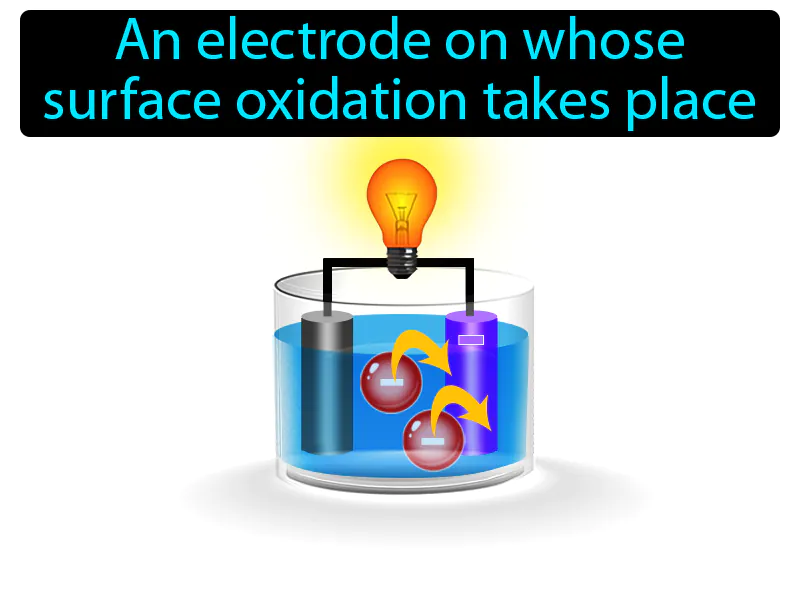
Anode: An electrode on whose surface oxidation takes place. anode. In simple terms, an anode is the part of a battery where electricity flows out, and chemical reactions take place.