Average Atomic Mass
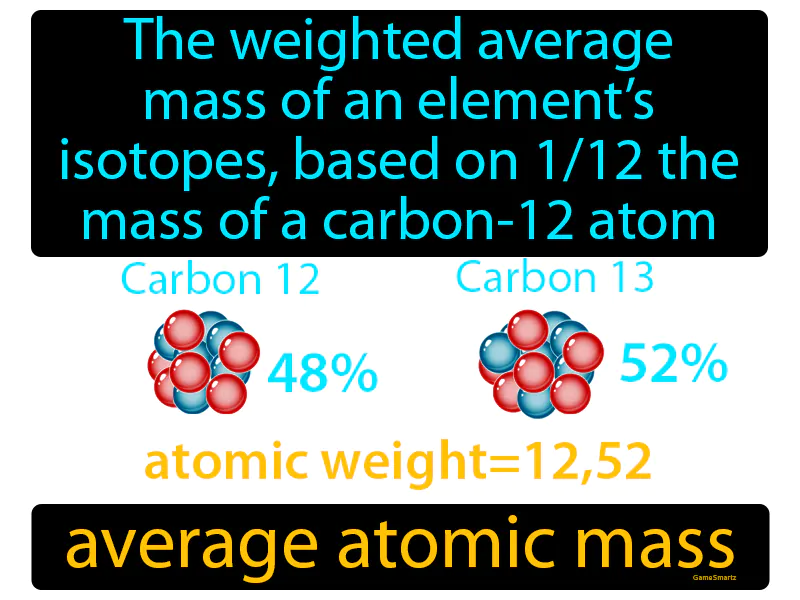
Imagine you're trying to calculate the average price of fruits in your basket, but you have different types of fruits with varying prices. This is similar to determining the average atomic mass of an element, where each isotope of the element has a different mass and abundance. Just as you need to consider both the price and the quantity of each type of fruit to get an accurate average cost, the average atomic mass is a weighted average based on the mass and relative abundance of each isotope, using the mass of a carbon-12 atom as a reference point.
Practice Version
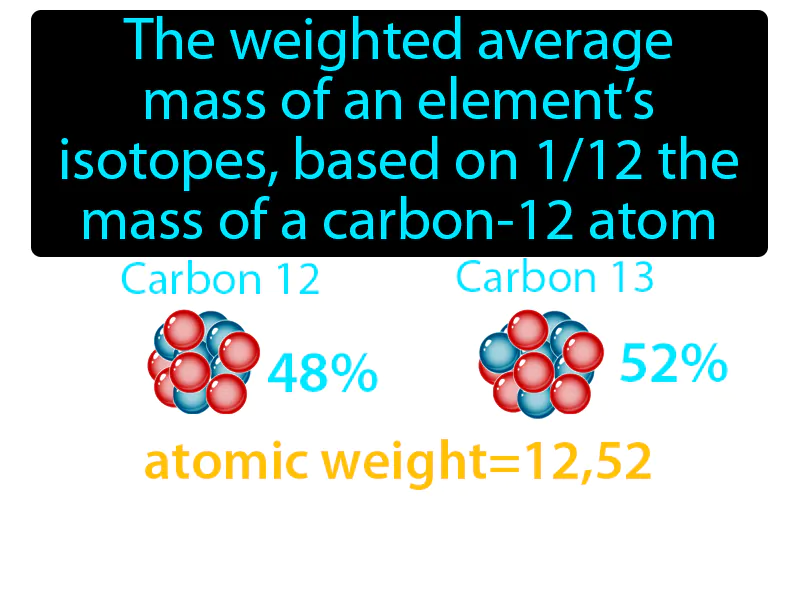
Average Atomic Mass: The weighted average mass of an elements isotopes, based on 112 the mass of a carbon-12 atom. Average atomic mass. Average atomic mass is the calculated mean mass of an element's isotopes, taking into account their natural abundance.