Atomic Mass Unit
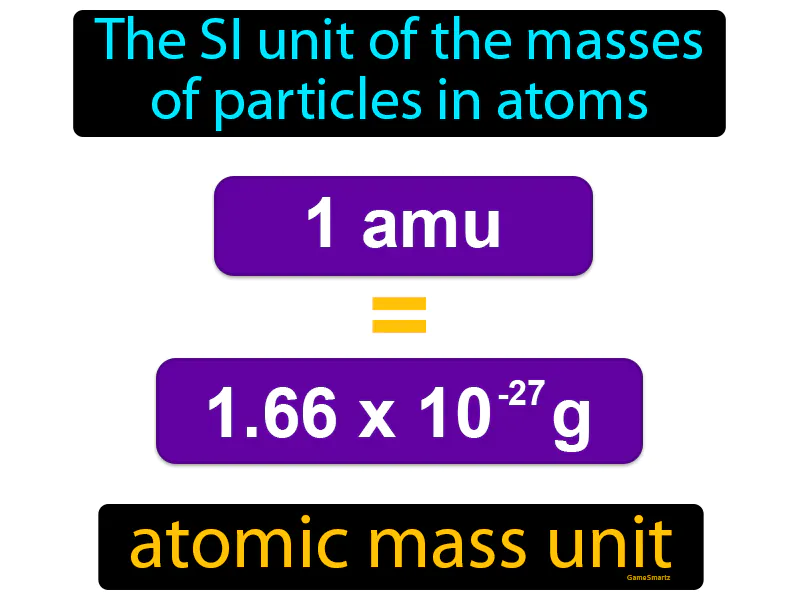
Imagine trying to weigh a single grain of sand on your bathroom scale. Just like it's challenging to measure something as tiny as a grain of sand with a scale meant for heavier objects, scientists face a similar challenge when trying to measure the mass of particles in atoms, which are incredibly small. The atomic mass unit (amu) acts like a specialized tool, much like a jeweler’s scale designed specifically to measure tiny grains, allowing scientists to precisely quantify the masses of atomic particles with appropriate accuracy.
Practice Version
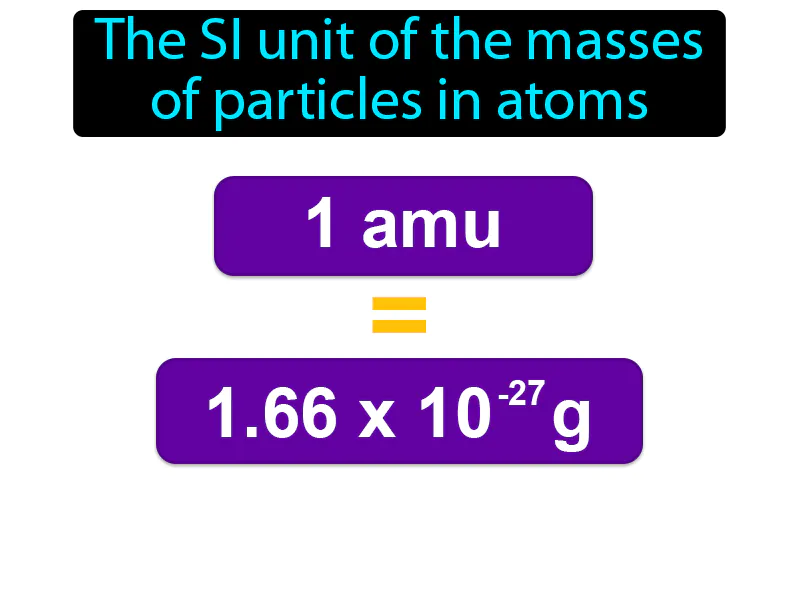
Atomic Mass Unit: The SI unit of the masses of particles in atoms atomic mass unit. An atomic mass unit amu is a small unit of mass used to measure the masses of particles in an atom, like protons and neutrons, where one amu is approximately 112 the mass of a carbon-12 atom.