Diatomic Molecule
Diatomic molecule explained in an easy to understand way:
Imagine trying to keep two magnets stuck together while they naturally want to either attract or repel each other. This is similar to how a diatomic molecule is formed by two atoms that bond together, sharing or exchanging electrons to maintain their connection. Just as the magnets need a balance of force to stay together, the two atoms in a diatomic molecule need a balance of energy, like a mutual agreement, to remain connected and stable.
Practice Version
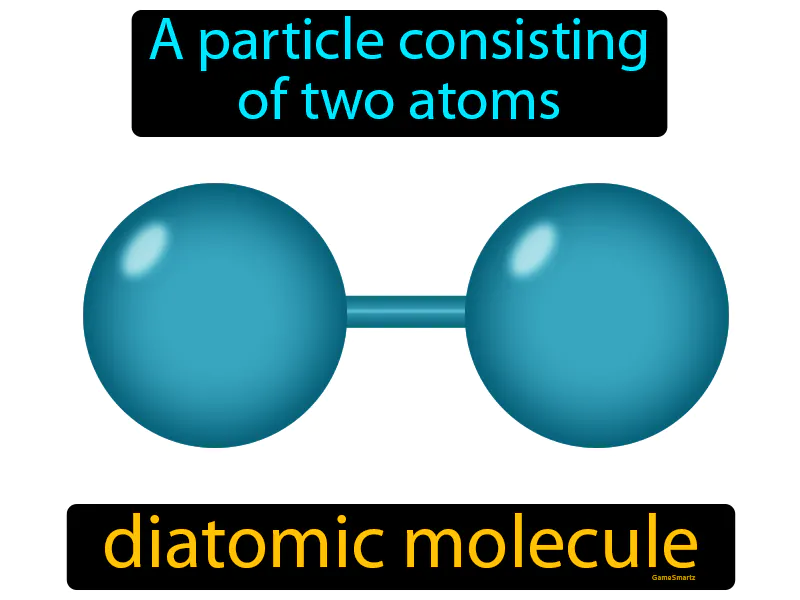
Diatomic Molecule: A particle consisting of two atoms. Diatomic molecule. A diatomic molecule is a molecule made up of two atoms bonded together, like oxygen O2 or hydrogen H2.